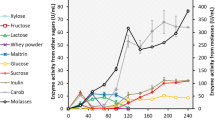
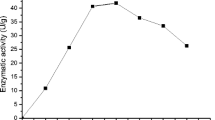
Abstract
Inulinases are commonly used for the production of high-fructose syrup, fructooligosaccharides, and inulooligosaccharides and also in the food and pharmaceutical industries. Besides, inulinases are also utilized for the fabrication of various high-added-value products such as bioethanol, lactic acid, citric acid, sugar alcohols, single-cell oils and proteins, 2,3-butanediol, gluconic acid, and butanol. Besides, before using an enzyme in a bioprocess, the determination of its properties is very essential. Therefore, this study evaluated the thermostability (30–80 °C for 0.5–6 h) of Aspergillus niger inulinase produced from sugar beet molasses in the shake flask fermentation and determined its kinetic and thermodynamic features. Based on the thermostability test of inulinase, it was stable at 30 °C and 40 °C and activity retained approximately 78.5% and 21.8% at 50 °C and 60 °C at the end of 6-h incubation time. Besides, half-life and D-value of enzyme reduced with increasing temperature, but increased with increasing incubation time at a fixed temperature. Q10-values were generally below one and reduced with increasing temperature. Nevertheless, inactivation energy and Z-value of inulinase were between 203.8 and 293.2 kJ/mol and between 17.05 and 20.99 °C to varying incubation times. Enthalpy and entropy of inulinase decreased with increasing temperature. The free energy of inulinase was unstable with increasing temperature depending on its inactivation energy. Consequently, inulinase can be used for biocatalytic strategies in various bioprocesses at relatively high temperatures.

Similar content being viewed by others
References
Yuan B, Hu N, Sun J, Wang S-A, Li F-L (2012) Purification and characterization of a novel extracellular inulinase from a new yeast species Candida kutaonensis sp. nov. KRF1T. Appl Microbiol Biotechnol 96(6):1517–1526
Singh R, Singh R (2017) Inulinases. In: Current developments in biotechnology and bioengineering. Elsevier, In, pp 423–446
Barthomeuf C, Regerat F, Pourrat H (1991) Production of inulinase by a new mold of Penicillium rugulosum. J Ferment Bioeng 72(6):491–494
Vandamme EJ, Derycke DG (1983) Microbial inulinases: fermentation process, properties, and applications. Adv Appl Microbiol 29:139–176
Zittan L (1981) Enzymatic hydrolysis of inulin-an alternative way to fructose production. Starch 33(11):373–377
Pandey A, Soccol CR, Selvakumar P, Soccol VT, Krieger N, Fontana JD (1999) Recent developments in microbial inulinases. Appl Biochem Biotechnol 81(1):35–52
Singh RS, Chauhan K, Pandey A, Larroche C, Kennedy JF (2018) Purification and characterization of two isoforms of exoinulinase from Penicillium oxalicum BGPUP-4 for the preparation of high fructose syrup from inulin. Int J Biol Macromol 118:1974–1983
Singh RS, Chauhan K, Kennedy JF (2017) A panorama of bacterial inulinases: production, purification, characterization and industrial applications. Int J Biol Macromol 96:312–322
Duruksu G, Ozturk B, Biely P, Bakir U, Ogel ZB (2009) Cloning, expression and characterization of endo-β-1,4-mannanase from Aspergillus fumigatus in Aspergillus sojae and Pichia pastoris. Biotechnol Prog 25(1):271–276
Germec M, Yatmaz E, Karahalil E, Turhan I (2017) Effect of different fermentation strategies on β-mannanase production in fed-batch bioreactor system. 3 Biotech 7:77
Jana UK, Suryawanshi RK, Prajapati BP, Soni H, Kango N (2018) Production optimization and characterization of mannooligosaccharide generating beta-mannanase from Aspergillus oryzae. Bioresour Technol 268:308–314
Karahalil E, Germec M, Karaoglan M, Yatmaz E, Coban HB, Inan M, Turhan I (2019) Partial purification and characterization of a recombinant β-mannanase from Aspergillus fumigatus expressed in Aspergillus sojae grown on carob extract. Biomass Conver Bio. https://doi.org/10.1007/s13399-019-00487-1
Karahalil E, Germec M, Turhan I (2019) β-Mannanase production and kinetic modeling from carob extract by using recombinant Aspergillus sojae. Biotechnol Prog 35:e2885. https://doi.org/10.1002/btpr.2885
Liu Z, Ning C, Yuan M, Yang S, Wei X, Xiao M, Fu X, Zhu C, Mou H (2020) High-level expression of a thermophilic and acidophilic beta-mannanase from Aspergillus kawachii IFO 4308 with significant potential in mannooligosaccharide preparation. Bioresour Technol 295:122257. https://doi.org/10.1016/j.biortech.2019.122257
Ozturk B, Cekmecelioglu D, Ogel ZB (2010) Optimal conditions for enhanced β-mannanase production by recombinant Aspergillus sojae. J Mol Catal B Enzym 64(3–4):135–139
Yatmaz E, Germec M, Karahalil E, Turhan I (2020) Enhancing β-mannanase production by controlling fungal morphology in the bioreactor with microparticle addition. Food Bioprod Process 121:123–130
Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan I (2016) Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng 39(9):1391–1399
Yatmaz E, Karahalil E, Germec M, Oziyci HR, Karhan M, Ogel ZB, Duruksu G, Turhan I (2016) Enhanced β-mannanase production from alternative sources by recombinant Aspergillus sojae. Acta Aliment 45(3):371–379
Yilmazer C, Germec M, Turhan I (2020) Solid-state fermentation for the production of a recombinant β-mannanase from Aspergillus fumigatus expressed in Aspergillus sojae grown on renewable resources. J Food Process Preserv. https://doi.org/10.1111/jfpp14584
Kalaskar VV, Kasinathan N, Subrahmanyam VM, Rao JV (2020) Optimization of extracellular acid protease production from Aspergillus niger by factorial design. J Microbiol Biotechnol Food Sci 9(4):132–136
Coban HB, Demirci A, Turhan I (2015) Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng 38(6):1075–1080
Coban HB, Demirci A, Turhan I (2015) Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng 38(8):1431–1436
Mandari V, Nema A, Devarai SK (2020) Sequential optimization and large scale production of lipase using tri-substrate mixture from Aspergillus niger MTCC 872 by solid state fermentation. Process Biochem 89:46–54
Karahalil E, Demirel F, Evcan E, Germeç M, Tari C, Turhan I (2017) Microparticle-enhanced polygalacturonase production by wild type Aspergillus sojae. 3 Biotech 7(6):361
Pasin TM, dos Anjos ME, de Lucas RC, Benassi VM, Ziotti LS, Cereia M, de Moraes MLT (2020) Novel amylase-producing fungus hydrolyzing wheat and brewing residues, Aspergillus carbonarius, discovered in tropical forest remnant. Folia Microbiol 65(1):173–184
Dong M, Wang S, Xiao G, Xu F, Hu W, Li Q, Chen J, Li W (2019) Cellulase production by Aspergillus fumigatus MS13. 1 mutant generated by heavy ion mutagenesis and its efficient saccharification of pretreated sweet sorghum straw. Process Biochem 84:22–29
de Souza JB, Michelin M, Amâncio FL, Brazil OAV, Maria de Lourdes T, Ruzene DS, Silva DP, Mendonça MC, López JA (2020) Sunflower stalk as a carbon source inductive for fungal xylanase production. Ind Crop Prod 153:112368
Germec M, Gürler HN, Ozcan A, Erkan SB, Karahalil E, Turhan I (2020) Medium optimization and kinetic modeling for the production of Aspergillus niger inulinase. Bioprocess Biosyst Eng 43(2):217–232
Germec M, Ozcan A, Turhan I (2019) Bioconversion of wheat bran into high value-added products and modelling of fermentations. Ind Crop Prod 139:111565
Germec M, Turhan I (2019) Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioprocess Biosyst Eng 42:1993–2005
Germec M, Turhan I (2020) Enhanced production of Aspergillus niger inulinase from sugar beet molasses and its kinetic modeling. Biotechnol Lett. https://doi.org/10.1007/s10529-020-02913-1
Gürler HN, Germec M, Turhan I (2020) The inhibition effect of phenol on the production of Aspergillus niger inulinase and its modeling. J Food Process Preserv. https://doi.org/10.1111/jfpp.14522
Ilgın M, Germec M, Turhan I (2020) Inulinase production and mathematical modeling from carob extract by using Aspergillus niger. Biotechnol Prog 36. https://doi.org/10.1002/btpr.2919
Das D, Bhat MR, Selvaraj R (2019) Review of inulinase production using solid-state fermentation. Ann Microbiol 69(3):201–209
Das D, Selvaraj R, Bhat MR (2019) Optimization of inulinase production by a newly isolated strain Aspergillus flavus var. flavus by solid state fermentation of Saccharum arundinaceum. Biocatal Agric Biotechnol 22:101363
Ongen-Baysal G, Sukan SS, Vassilev N (1994) Production and properties of inulinase from Aspergillus niger. Biotechnol Lett 16(3):275–280
Cemeroğlu B (2015) Reaksiyon Kinetiği. Bizim Grup Basımevi, Ankara
Pal A, Khanum F (2011) Characterizing and improving the thermostability of purified xylanase from Aspergillus niger DFR-5 grown on solid-state-medium. J Biochem Technol 2(4):203–209
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Flores-Gallegos AC, Contreras-Esquivel JC, Aguilar CN (2015) Comparative study of fungal strains for thermostable inulinase production. J Biosci Bioeng 119(4):421–426
Nakamura T, Ogata Y, Shitara A, Nakamura A, Ohta K (1995) Continuous production of fructose syrups from inulin by immobilized inulinase from Aspergillus niger mutant 817. J Ferment Bioeng 80(2):164–169
Shkutina I, Stoyanova O, Selemenev V (2016) Biocatalytic properties of immobilized inulinase. Russ J Bioorg Chem 42(7):748–751
Zhao Z, Zhao Z, Wang X (2016) Kinetic and thermodynamic characterizations of thermal inactivation of the inulinase produced by Kluyveromyces latics. Paper presented at the 5th International Conference on Advanced Materials and Computer Science, Beijing, China,
Tunail N (2009) Biyoenerjetiğin pirensipleri ve metabolizmaya giriş. In: Tunail N (ed) Mikrobiyoloji. Pelin Ofset, Ankara, pp 229–264
Torabizadeh H, Habibi-Rezaei M, Safari M, Moosavi-Movahedi AA, Sharifizadeh A, Azizian H, Amanlou M (2011) Endo-inulinase stabilization by pyridoxal phosphate modification: a kinetics, thermodynamics, and simulation approach. Appl Biochem Biotechnol 165(7–8):1661–1673
Acknowledgments
This work was supported by the Akdeniz University Research Foundation (Grant number #FDK-2019-4761).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Germec, M., Turhan, I. Thermostability of Aspergillus niger inulinase from sugar beet molasses in the submerged fermentation and determination of its kinetic and thermodynamic parameters. Biomass Conv. Bioref. 12, 3219–3227 (2022). https://doi.org/10.1007/s13399-020-00809-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00809-8