Abstract
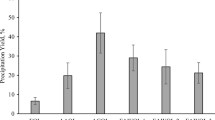
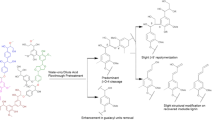
The solvolysis of technical lignins was evaluated using water/ethanol solvent mixture with the aim of producing monomeric aromatic compounds in a continuous flow bench reactor. Four lignins issued from various biomasses (softwood, wheat straw) and extracted using different pulping processes (kraft, soda, organosolv) were engaged in the study. Before being converted, these lignins (two kraft, a soda, and an organosolv) were characterized by a multitechnique approach coupling compositional analysis, NMR, IR, GC, and SEC. Data thus obtained allowed us to point out the main differences in terms of structure and chemical composition. Based on its high solubility in water and water/ethanol mixture, alkaline lignin (i.e., from the kraft process) was used to optimize the reaction parameters. Thus, we found out that working at 225 °C under 8 MPa nitrogen using a 1-wt% basic solution of lignin allowed to operate the reactor without difficulties (no foam formation, no char) when a 1/1 water/ethanol mixture was used. Under these conditions, alkaline lignin was converted up to 76%. Other lignins were then evaluated leading to conversions between 46 and 74%. The reaction mixture obtained was fractioned to PM (precipitated matter), OP (organic products), and AP (aqueous phase soluble products) to allow careful analyses of the products. Analytical data obtained showed particularly that the number of β-O-4 linkages in the remaining solid material (PM) decreases significantly (up to − 95%) that correlated with the formation of monomeric organic compounds in OP, however in low amount due to over reactions leading to compounds evolving in the aqueous phase. Generally, whatever the lignin, guaiacol is the main aromatic compound observed under all reaction conditions evaluated.







Similar content being viewed by others
References
Ragauskas AJ (2006) The path forward for biofuels and biomaterials. Science 311(80):484–489. https://doi.org/10.1126/science.1114736
Vassilev SV, Baxter D, Andersen LK, Vassileva CG, Morgan TJ (2012) An overview of the organic and inorganic phase composition of biomass. Fuel 94:1–33. https://doi.org/10.1016/j.fuel.2011.09.030
Terashima N (1989) An improved radiotracer method for studying formation and structure of lignin. pp 148–159
Parthasarathi R, Romero RA, Redondo A, Gnanakaran S (2011) Theoretical study of the remarkably diverse linkages in lignin. J Phys Chem Lett 2:2660–2666. https://doi.org/10.1021/jz201201q
Vishtal A, Kraslawski A (2011) Challenges in industrial applications of technical lignins. BioResources 6:3547–3568. https://doi.org/10.15376/BIORES.6.3.3547-3568
Constant S, Wienk HLJ, Frissen AE, Peinder P, Boelens R, van Es DS, Grisel RJH, Weckhuysen BM, Huijgen WJJ, Gosselink RJA, Bruijnincx PCA (2016) New insights into the structure and composition of technical lignins: a comparative characterisation study. Green Chem 18:2651–2665. https://doi.org/10.1039/C5GC03043A
Gosselink RJA, de Jong E, Guran B, Abächerli A (2004) Co-ordination network for lignin—standardisation, production and applications adapted to market requirements (EUROLIGNIN). Ind Crop Prod 20:121–129. https://doi.org/10.1016/j.indcrop.2004.04.015
Botello JI, Gilarranz MA, Rodríguez F, Oliet M (1999) Preliminary study on products distribution in alcohol pulping of Eucalyptus globulus. J Chem Technol Biotechnol 74:141–148. https://doi.org/10.1002/(SICI)1097-4660(199902)74:2<141::AID-JCTB1>3.0.CO;2-0
Wildschut J, Smit AT, Reith JH, Huijgen WJJ (2013) Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour Technol 135:58–66. https://doi.org/10.1016/j.biortech.2012.10.050
Quesada-Medina J, López-Cremades FJ, Olivares-Carrillo P (2010) Organosolv extraction of lignin from hydrolyzed almond shells and application of the δ-value theory. Bioresour Technol 101:8252–8260. https://doi.org/10.1016/j.biortech.2010.06.011
Pan X (2012) Organosolv biorefining platform for producing chemicals, fuels, and materials from lignocellulose. In: The role of green chemistry in biomass processing and conversion. Wiley, Hoboken, pp 241–262
Nitsos C, Stoklosa R, Karnaouri A, Vörös D, Lange H, Hodge D, Crestini C, Rova U, Christakopoulos P (2016) Isolation and characterization of organosolv and alkaline lignins from hardwood and softwood biomass. ACS Sustain Chem Eng 4:5181–5193. https://doi.org/10.1021/acssuschemeng.6b01205
Oliet M, Garcia J, Rodriguez F, Gilarrranz MA (2002) Solvent effects in autocatalyzed alcohol–water pulping: comparative study between ethanol and methanol as delignifying agents. Chem Eng J 87:157–162. https://doi.org/10.1016/S1385-8947(01)00213-3
McDonough TJ (1992) The chemistry of organosolv delignification
Huang X, Korányi TI, Boot MD, Hensen EJM (2014) Catalytic depolymerization of lignin in supercritical ethanol. ChemSusChem 7:2276–2288. https://doi.org/10.1002/cssc.201402094
Miller JE, Evans L, Littlewolf A, Trudell DE (1999) Batch microreactor studies of lignin and lignin model compound depolymerization by bases in alcohol solvents. Fuel 78:1363–1366. https://doi.org/10.1016/S0016-2361(99)00072-1
Voitl T, Von Rohr PR (2010) Demonstration of a process for the conversion of kraft lignin into vanillin and methyl vanillate by acidic oxidation in aqueous methanol. Ind Eng Chem Res 49:520–525. https://doi.org/10.1021/ie901293p
Huang X, Korányi TI, Boot M, Hensen EJM (2015) Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem 17:4941–4950. https://doi.org/10.1039/C5GC01120E
Song Q, Wang F, Cai J, Wang Y, Zhang J, Yu W, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6:994. https://doi.org/10.1039/c2ee23741e
Abdelaziz OY, Li K, Tunå P, Hulteberg CP (2018) Continuous catalytic depolymerisation and conversion of industrial kraft lignin into low-molecular-weight aromatics. Biomass Convers Biorefinery 8:455–470. https://doi.org/10.1007/s13399-017-0294-2
Beauchet R, Monteil-Rivera F, Lavoie JM (2012) Conversion of lignin to aromatic-based chemicals (L-chems) and biofuels (L-fuels). Bioresour Technol 121:328–334. https://doi.org/10.1016/j.biortech.2012.06.061
Schmiedl D, Endisch S, Pindel E et al (2012) Base catalyzed degradation of lignin for the generation of oxy-aromatic compounds—possibilities and challenges. Erdöl Erdgas Kohl 10:357–363
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PCA, Weckhuysen BM (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chemie Int Ed 55:8164–8215. https://doi.org/10.1002/anie.201510351
Xu C, Arancon RAD, Labidi J, Luque R (2014) Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem Soc Rev 43:7485–7500. https://doi.org/10.1039/C4CS00235K
Zhang Z, Song J, Han B (2017) Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem Rev 117:6834–6880. https://doi.org/10.1021/acs.chemrev.6b00457
Gillet S, Aguedo M, Petitjean L, Morais ARC, da Costa Lopes AM, Łukasik RM, Anastas PT (2017) Lignin transformations for high value applications: towards targeted modifications using green chemistry. Green Chem 19:4200–4233. https://doi.org/10.1039/C7GC01479A
Kozliak EI, Kubátová A, Artemyeva AA, Nagel E, Zhang C, Rajappagowda RB, Smirnova AL (2016) Thermal liquefaction of lignin to aromatics: efficiency, selectivity, and product analysis. ACS Sustain Chem Eng 4:5106–5122. https://doi.org/10.1021/acssuschemeng.6b01046
Li S-H, Liu S, Colmenares JC, Xu Y-J (2016) A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem 18:594–607. https://doi.org/10.1039/C5GC02109J
Ma R, Guo M, Zhang X (2018) Recent advances in oxidative valorization of lignin. Catal Today 302:50–60. https://doi.org/10.1016/j.cattod.2017.05.101
Otromke M, White RJ, Sauer J (2019) Hydrothermal base catalyzed depolymerization and conversion of technical lignin—an introductory review. Carbon Resour Convers 2:59–71. https://doi.org/10.1016/j.crcon.2019.01.002
Sluiter A, Hames B, Ruiz R, et al (2012) Determination of structural carbohydrates and lignin in biomass
Crestini C, Argyropoulos DS (1997) Structural analysis of wheat straw lignin by quantitative 31P and 2D NMR spectroscopy. The occurrence of ester bonds and α-O-4 substructures. J Agric Food Chem 45:1212–1219
Granata A, Argyropoulos DS (1995) 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J Agric Food Chem 43:1538–1544
Faix O (1992) Fourier transform infrared spectroscopy. In: Lin SY, Dence CW (eds) Methods in lignin chemistry, 1st ed. Springer Series in Wood Science, pp 83–109
Pu Y, Cao S, Ragauskas AJ (2011) Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ Sci 4:3154. https://doi.org/10.1039/c1ee01201k
Guerra A, Norambuena M, Freer J, Argyropoulos DS (2008) Determination of arylglycerol−β-aryl ether linkages in enzymatic mild acidolysis lignins (EMAL): comparison of DFRC/31P NMR with thioacidolysis⊥. J Nat Prod 71:836–841. https://doi.org/10.1021/np800080s
Käldström M, Meine N, Farès C, Schüth F, Rinaldi R (2014) Deciphering ‘water-soluble lignocellulose’ obtained by mechanocatalysis: new insights into the chemical processes leading to deep depolymerization. Green Chem 16:3528–3538. https://doi.org/10.1039/C4GC00004H
Mansfield SD, Kim H, Lu F, Ralph J (2012) Whole plant cell wall characterization using solution-state 2D NMR. Nat Protoc 7:1579–1589. https://doi.org/10.1038/nprot.2012.064
Wen J-L, Sun S-L, Xue B-L, Sun R-C (2013) Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials (Basel) 6:359–391. https://doi.org/10.3390/ma6010359
Ni Y, Hu Q (1995) Alcell® lignin solubility in ethanol–water mixtures. J Appl Polym Sci 57:1441–1446. https://doi.org/10.1002/app.1995.070571203
Cheng S, D’cruz I, Wang M, Leitch M, Xu C(C) (2010) Highly efficient liquefaction of woody biomass in hot-compressed alcohol−water co-solvents†. Energy Fuel 24:4659–4667. https://doi.org/10.1021/ef901218w
Ye Y, Zhang Y, Fan J, Chang J (2012) Novel method for production of phenolics by combining lignin extraction with lignin depolymerization in aqueous ethanol. Ind Eng Chem Res 51:103–110. https://doi.org/10.1021/ie202118d
Ndou A (2003) Dimerisation of ethanol to butanol over solid-base catalysts. Appl Catal A Gen 251:337–345. https://doi.org/10.1016/S0926-860X(03)00363-6
Ferrini P, Rinaldi R (2014) Catalytic biorefining of plant biomass to non-pyrolytic lignin bio-oil and carbohydrates through hydrogen transfer reactions. Angew Chemie - Int Ed 53:8634–8639. https://doi.org/10.1002/anie.201403747
Gabriëls D, Hernández WY, Sels B, van der Voort P, Verberckmoes A (2015) Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal Sci Technol 5:3876–3902. https://doi.org/10.1039/C5CY00359H
Veibel S, Nielsen JI (1967) On the mechanism of the Guerbet reaction. Tetrahedron 23:1723–1733. https://doi.org/10.1016/S0040-4020(01)82571-0
Yang C, Meng ZY (1993) Bimolecular condensation of ethanol to 1-butanol catalyzed by alkali cation zeolites. J Catal 142:37–44. https://doi.org/10.1006/jcat.1993.1187
Chakar FS, Ragauskas AJ (2004) Review of current and future softwood kraft lignin process chemistry. Ind Crop Prod 20:131–141. https://doi.org/10.1016/j.indcrop.2004.04.016
Joffres B, Lorentz C, Vidalie M, Laurenti D, Quoineaud AA, Charon N, Daudin A, Quignard A, Geantet C (2014) Catalytic hydroconversion of a wheat straw soda lignin: characterization of the products and the lignin residue. Appl Catal B Environ 145:167–176. https://doi.org/10.1016/j.apcatb.2013.01.039
Lapierre C, Pollet B, Petit-Conil M, Toval G, Romero J, Pilate G, Leplé JC, Boerjan W, Ferret V, de Nadai V, Jouanin L (1999) Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol 119:153–164. https://doi.org/10.1104/pp.119.1.153
Delmas G-H (2011) La Biolignine™: Structure et Application à l’élaboration de résines époxy
Jongerius AL, Bruijnincx PCA, Weckhuysen BM (2013) Liquid-phase reforming and hydrodeoxygenation as a two-step route to aromatics from lignin. Green Chem 15:3049–3056. https://doi.org/10.1039/C3GC41150H
Funding
The authors gratefully acknowledge the National Agency of Research (CHEMLIVAL No. ANR-12-CDII-0001_01) for funding. W.S. thanks the French Ministry of Research and Education for the PhD grant.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2.44 mb)
Rights and permissions
About this article
Cite this article
Sebhat, W., El-Roz, A., Crepet, A. et al. Comparative study of solvolysis of technical lignins in flow reactor. Biomass Conv. Bioref. 10, 351–366 (2020). https://doi.org/10.1007/s13399-019-00435-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00435-z