Abstract
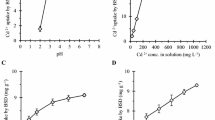
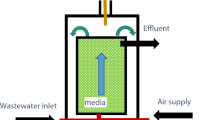
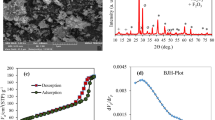
Ornidazole is a well-known antibiotic which has been widely used for both human and veterinary treatments. The present study investigated the degradation of ornidazole using \(\hbox {TiO}_{2}\) as a photocatalyst with UV light irradiation. Artificial neural network (ANN) was applied for the modeling of the photocatalytic degradation of ornidazole. In slurry mode, the input parameters were pH, ornidazole concentration, \(\hbox {TiO}_{2}\) dose, treatment time and % degradation as output. Parametric optimization was performed by Box–Behnken design (BBD). At optimum conditions the % degradation was found to be 84.02, 82.63 and 77.7% as predicted by BBD, simulated by ANN and by experimental run respectively. The results showed that the predictions agreed with the experimental results. The degradation of ornidazole follows the second-order reaction kinetics. For fixed-bed studies, \(\hbox {TiO}_{2}\) immobilized spherical cement beads were used to carry out the degradation of ornidazole at laboratory scale as well as at pilot scale with volume handling of 5 L. The catalyst immobilized beads were successfully recycled for at-least 40 runs without any significant reduction in the degradation efficiency of ornidazole. The activity as well as stability of immobilized catalyst over the surface of beads was confirmed through SEM/EDS, XRD and DRS analysis. Bioassay test was conducted for the safe disposal of treated wastewater and was found to be non-toxic.
Similar content being viewed by others
References
Dinh, Q.T.; Alliot, F.; Moreau-Guigon, E.; Eurin, J.J.; Chevreuil, M.; Labadie, P.: Measurement of trace levels of antibiotics in river water using on-line enrichment and triple-quadrupole LC–MS/MS. Talanta 85, 1238–1245 (2011). https://doi.org/10.1016/j.talanta.2011.05.013
Yang, L.; Yu, L.E.; Ray, M.B.: Degradation of paracetamol in aqueous solutions by \(\text{ TiO }_{2}\) photocatalysis. Water Res. 42, 3480–3488 (2008). https://doi.org/10.1016/j.watres.2008.04.023
Braz, F.; Silva, M.; Silva, F.: Photocatalytic degradation of ibuprofen using \(\text{ TiO }_{2}\) and ecotoxicological assessment of degradation intermediates against Daphnia similis. J. Environ. Prot. (Irvine, Calif) 5, 620–626 (2014)
Kümmerer, K.: Chemosphere antibiotics in the aquatic environment—a review—Part II. Chemosphere 75, 435–441 (2009). https://doi.org/10.1016/j.chemosphere.2008.12.006
Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Aguera, a; Fernandez-Alba, A.R.: Degradation of fifteen emerging contaminants at \(\upmu \text{ g }~\text{ L }^{-1}\) initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 44, 545–554 (2010). https://doi.org/10.1016/j.watres.2009.09.059
Sang, Z.; Jiang, Y.; Tsoi, Y.K.; Leung, K.S.Y.: Evaluating the environmental impact of artificial sweeteners: a study of their distributions, photodegradation and toxicities. Water Res. 52, 260–264 (2014). https://doi.org/10.1016/j.watres.2013.11.002
Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H.: Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 39, 3139–3152 (2005). https://doi.org/10.1016/j.watres.2005.05.031
Teixeira, S.; Gurke, R.; Eckert, H.; Kuhn, K.; Fauler, J.; Cuniberti, G.: Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions. J. Environ. Chem. Eng. 4, 287–292 (2016). https://doi.org/10.1016/j.jece.2015.10.045
Ziemiańska, J.; Adamek, E.; Sobczak, A.; Lipska, I.; Makowski, A.; Baran, W.: The study of photocatalytic degradation of sulfonamides applied to municipal wastewater. Physicochem. Probl. Miner. Process. 45, 127–140 (2010)
Giri, R.R.; Ozaki, H.; Ota, S.; Takanami, R.; Taniguchi, S.: Degradation of common pharmaceuticals and personal care products in mixed solutions by advanced oxidation techniques. Int. J. Environ. Sci. Technol. 7, 251–260 (2010). https://doi.org/10.1007/BF03326135
Kim, I.; Tanaka, H.: Photodegradation characteristics of PPCPs in water with UV treatment. Environ. Int. 35, 793–802 (2009). https://doi.org/10.1016/j.envint.2009.01.003
Fujishima, A.; Rao, T.N.; Tryk, D.A.: Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 1, 1–21 (2000). https://doi.org/10.1016/S1389-5567(00)00002-2
Mills, A.; Le Hunte, S.: An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 108, 1–35 (1997). https://doi.org/10.1016/S1010-6030(97)00118-4
Fox, M.A.; Dulay, M.T.: Heterogeneous photocatalysis. Chem. Rev. 93, 341–357 (1993). https://doi.org/10.1021/cr00017a016
Palmisano, G.; García-López, E.; Marcì, G.; Loddo, V.; Yurdakal, S.; Augugliaro, V.; Palmisano, L.: Advances in selective conversions by heterogeneous photocatalysis. Chem. Commun. (Cambr) 46, 7074–89 (2010). https://doi.org/10.1039/c0cc02087g
Fujishima, A.; Zhang, X.; Tryk, D.A.: TiO\(_{2}\) Photocatalysis and Related Surface Phenomena. Elsevier, Amsterdam (2008)
ArunaKumari, M.L.; Devi, L.G.: New insights into the origin of the visible light photocatalytic activity of Fe (III) porphyrin surface anchored \(\text{ TiO }_{2}\). Environ. Sci. 1, 177–187 (2015). https://doi.org/10.1039/C4EW00024B
Zheng, Q.; Shen, H.; Shuai, D.: Emerging investigators series: advances and environmental science challenges of graphitic carbon nitride as a visible. Environ. Sci. Water Res. Technol. 3, 982 (2017). https://doi.org/10.1039/C7EW00159B
Pistkova, V.; Tasbihi, M.; Vavrova, M.; Stangar, U.L.: Photocatalytic degradation of \(\beta \)-blockers by using immobilized titania/silica on glass slides. J. Photochem. Photobiol. A Chem. 305, 19–28 (2015). https://doi.org/10.1016/j.jphotochem.2015.02.014
Prieto-Rodriguez, L.; Miralles-Cuevas, S.; Oller, I.; Aguera, a; Puma, G.L.; Malato, S.: Treatment of emerging contaminants in wastewater treatment plants (WWTP) effluents by solar photocatalysis using low \(\text{ TiO }_{2}\) concentrations. J. Hazard. Mater. 211–212, 131–137 (2012). https://doi.org/10.1016/j.jhazmat.2011.09.008
Sarkar, S.; Chakraborty, S.; Bhattacharjee, C.: Photocatalytic degradation of pharmaceutical wastes by alginate supported \(\text{ TiO }_{2}\) nanoparticles in packed bed photo reactor (PBPR). Ecotoxicol. Environ. Saf. 121, 263–270 (2015). https://doi.org/10.1016/j.ecoenv.2015.02.035
Zhao, J.; Yao, B.; He, Q.; Zhang, T.: Preparation and properties of visible light responsive \(\text{ Y }^{3+}\) doped \(\text{ Bi }_{5}\text{ Nb }_{3}\text{ O }_{15}\) photocatalysts for Ornidazole decomposition. J. Hazard. Mater. 229–230, 151–158 (2012). https://doi.org/10.1016/j.jhazmat.2012.05.088
Fukahori, S.; Fujiwara, T.: Photocatalytic decomposition behavior and reaction pathway of sulfamethazine antibiotic using \(\text{ TiO }_{2}\). J. Environ. Manag. 157, 103–110 (2015). https://doi.org/10.1016/j.jenvman.2015.04.002
Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M.: Photocatalytic degradation of paracetamol on \(\text{ TiO }_{2}\)nanoparticles and \(\text{ TiO }_{2}\)/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem. 10, S3640–S3645 (2017). https://doi.org/10.1016/j.arabjc.2014.03.014
Rosu, M.C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S.: Azo dyes degradation using \(\text{ TiO }_{2}\)-Pt/graphene oxide and \(\text{ TiO }_{2}\)-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 70, 13–20 (2017). https://doi.org/10.1016/j.solidstatesciences.2017.05.013
Talwar, S.; Sangal, V.K.; Verma, A.: Feasibility of using combined \(\text{ TiO }_{2}\) photocatalysis and RBC process for the treatment of real pharmaceutical wastewater. J. Photochem. Photobiol. A Chem. 353, 119 (2018). https://doi.org/10.1016/j.jphotochem.2017.11.013
Aleboyeh, A.; Moussa, Y.; Aleboyeh, H.: The effect of operational parameters on UV/\(\text{ H }_{2}\text{ O }_{2}\) decolourisation of Acid Blue 74. Dye Pigment. 66, 129–134 (2005). https://doi.org/10.1016/j.dyepig.2004.09.008
Verma, A.; Prakash, N.T.; Toor, A.P.: An efficient \(\text{ TiO }_{2}\) coated immobilized system for the degradation studies of herbicide isoproturon: durability studies. Chemosphere 109, 7–13 (2014). https://doi.org/10.1016/j.chemosphere.2014.02.051
Garg, A.; Sangal, V.K.; Bajpai, P.K.: Decolorization and degradation of reactive black 5 dye by photocatalysis: modeling, optimization and kinetic study. Desalin. Water Treat. 3994, 1 (2015). https://doi.org/10.1080/19443994.2015.1086697
Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Artur, S.J.: Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 38, 369–380 (2007). https://doi.org/10.1590/S1517-83822007000200034
Elmolla, E.S.; Chaudhuri, M.; Eltoukhy, M.M.: The use of artificial neural network (ANN) for modeling of COD removal from antibiotic aqueous solution by the Fenton process. J. Hazard. Mater. 179, 127–134 (2010). https://doi.org/10.1016/j.jhazmat.2010.02.068
Zamaniyan, A.; Joda, F.; Behroozsarand, A.; Ebrahimi, H.: Application of artificial neural networks (ANN) for modeling of industrial hydrogen plant. Int. J. Hydrog. Energy 38, 6289–6297 (2013). https://doi.org/10.1016/j.ijhydene.2013.02.136
Kaur, P.; Sangal, V.K.; Kushwaha, J.P.: Modeling and evaluation of electro-oxidation of dye wastewater using artificial neural networks. RSC Adv. 5, 34663–34671 (2015). https://doi.org/10.1039/c4ra14160a
Bhatti, M.S.; Kapoor, D.; Kalia, R.K.; Reddy, A.S.; Thukral, A.K.: RSM and ANN modeling for electrocoagulation of copper from simulated wastewater: multi objective optimization using genetic algorithm approach. Desalination 274, 74–80 (2011). https://doi.org/10.1016/j.desal.2011.01.083
Agatonovic-Kustrin, S.; Beresford, R.: Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 22, 717–727 (2000). https://doi.org/10.1016/S0731-7085(99)00272-1
Pareek, V.; Brungs, M.; Adesina, a; Sharma, R.: Artificial neural network modeling of a multiphase photodegradation system. J. Photochem. Photobiol. A Chem. 149, 139–146 (2002). https://doi.org/10.1016/S1010-6030(01)00640-2
Sangal, V.K.; Kumar, V.; Mishra, I.M.: Process parametric optimization of a divided wall distillation column. Chem. Eng. Commun. 201, 37–41 (2014). https://doi.org/10.1080/00986445.2012.762625
Rastegar, M.; Shadbad, K.R.; Khataee, A.R.; Pourrajab, R.: Optimization of photocatalytic degradation of sulphonated diazo dye C.I. reactive green 19 using ceramic-coated TiO\(_{2}\) nanoparticles. Environ. Technol. 33, 995–1003 (2012). https://doi.org/10.1080/09593330.2011.604859
Singh, P.; Mittal, R.; Sharma, G.C.; Singh, S.; Singh, A.: Ornidazole: comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 30, 123–184 (2003)
Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C.: Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 16, 10370–10386 (2011). https://doi.org/10.3390/molecules161210370
Rizzo, L.: Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 45, 4311–4340 (2011). https://doi.org/10.1016/j.watres.2011.05.035
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talwar, S., Sangal, V.K., Verma, A. et al. Modeling, Optimization and Kinetic Study for Photocatalytic Treatment of Ornidazole Using Slurry and Fixed-Bed Approach. Arab J Sci Eng 43, 6191–6202 (2018). https://doi.org/10.1007/s13369-018-3388-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-018-3388-7