Abstract
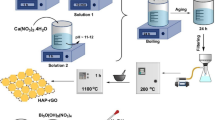
The excessive phosphate discharged into the environment has damaged the ecosystem seriously. In this work, a kind of 3D adsorbents, \(\upalpha \hbox {-Fe}_{2}\hbox {O}_{3}\) decorated graphene oxide \((\hbox {GO--Fe}_{2}\hbox {O}_{3})\), was synthesized to deal with phosphate-polluted water. The phosphate adsorption capacity of \(\hbox {GO--Fe}_{2}\hbox {O}_{3}\) reached to \(93.28\,\hbox { mg\,g}^{-1}\), in \(50\,\hbox { mg\, L}^{-1}\) phosphate solution at pH 6.0 and temperature 298 K, and the phosphate adsorption efficiency is very stable between the pH range of 2.0–10.5 and temperature range of 293–333 K. The adsorption progress is rapid, and adsorption equilibrium was reached within 5 min. The phosphate adsorption behavior of \(\hbox {GO--Fe}_{2}\hbox {O}_{3}\) fitted the Langmuir model, and the adsorption kinetic fitted the pseudo-second-order model. Ion exchange and electrostatic attraction are the main reactions in the adsorption process. Physical adsorption and chemical adsorption both are in the adsorption process. The phosphate adsorption progress is stable and rapid; thus, it is a good choice to deal with phosphate-polluted water by \(\hbox {GO--Fe}_{2}\hbox {O}_{3}\).
Similar content being viewed by others
References
Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A.: Improved phosphorus use efficiency in agriculture: a key requirement for its sustainable use. Chemosphere 84(6), 822–831 (2011)
Pieterse, N.; Bleuten, W.; Jørgensen, S.: Contribution of point sources and diffuse sources to nitrogen and phosphorus loads in lowland river tributaries. J. Hydrol. 271(1), 213–225 (2003)
Arvin, E.: Observations supporting phosphate removal by biologically mediated chemical precipitation. Rev. Water Sci. Technol. 15(3/4), 43–63 (2011)
Paul, D.; Sinha, S.N.: Biological removal of phosphate using phosphate solubilizing bacterial consortium from synthetic wastewater: a laboratory scale. Environmentasia 8(1), 1–8 (2015)
Xu, P.; Capito, M.; Cath, T.Y.: Selective removal of arsenic and monovalent ions from brackish water reverse osmosis concentrate. J. Hazard. Mater. 260(18), 885–891 (2013)
Yang, Y.; Lohwacharin, J.; Takizawa, S.: Hybrid ferrihydrite-MF/UF membrane filtration for the simultaneous removal of dissolved organic matter and phosphate. Water Res. 65, 177–185 (2014)
Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R.: Development of constructed wetlands inperformance intensifications for wastewater treatment: a nitrogen and organic matter targeted review. Water Res. 57C(5), 40–55 (2014)
Kulkarni, S.J.: Advancements, research and challenges in reactive adsorption: a review. Int. J. Res. 2(1), 477–480 (2015)
Ye, J.; Cong, X.; Zhang, P.; Hoffmann, E.; Zeng, G.; Liu, Y.; Fang, W.; Wu, Y.; Zhang, H.: Interaction between phosphate and acid-activated neutralized red mud during adsorption process. Appl. Surf. Sci. 356, 128–134 (2015)
Rui, M.N.; Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A.: Novel porous fly-ash containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard. Mater. 318, 631 (2016)
Apul, O.G.; Wang, Q.; Zhou, Y.; Karanfil, T.: Adsorption of aromatic organic contaminants by graphene nanosheets: comparison with carbon nanotubes and activated carbon. Water Res. 47(4), 1648–1654 (2013)
And, C.C.; Wang, X.: Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind. Eng. Chem. Res. 45(26), 9144–9149 (2015)
Rimola, A.; Costa, D.; Sodupe, M.; Lambert, J.-F.; Ugliengo, P.: Silica surface features and their role in the adsorption of biomolecules: computational modeling and experiments. Chem. Rev. 113(6), 4216–4313 (2013)
Panic, V.V.; Velickovic, S.J.: Removal of model cationic dye by adsorption onto poly (methacrylic acid)/zeolite hydrogel composites: kinetics, equilibrium study and image analysis. Sep. Purif. Technol. 122, 384–394 (2014)
Xie, J.; Wang, Z.; Fang, D.; Li, C.; Wu, D.: Green synthesis of a novel hybrid sorbent of zeolite/lanthanum hydroxide and its application in the removal and recovery of phosphate from water. J. Colloid Interf. Sci. 423, 13–19 (2014)
Cao, C.Y.; Qu, J.; Yan, W.S.; Zhu, J.F.; Wu, Z.Y.; Song, W.G.: Low-cost synthesis of flowerlike \(\alpha \text{-Fe }_{2}\text{ O }_{3}\) nanostructures for heavy metal ion removal: adsorption property and mechanism. Langmuir 28(9), 4573–4579 (2012)
Kakavandi, B.; Kalantary, R.R.; Jafari, A.J.; Nasseri, S.; Ameri, A.; Esrafili, A.; Azari, A.: Pb(II) adsorption onto a magnetic composite of activated carbon and superparamagnetic \(\text{ Fe }_{3}\text{ O }_{4}\) nanoparticles: experimental and modeling study. CLEAN Soil Air Water 43(8), 1157–1166 (2015)
Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S.: Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl. Mater. Interf. 4(3), 1186–1193 (2012)
Najafi, F.; Moradi, O.; Rajabi, M.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K.: Thermodynamics of the adsorption of nickel ions from aqueous phase using graphene oxide and glycine functionalized graphene oxide. J. Mol. Liq. 208, 106–113 (2015)
Mohan, S.; Kumar, V.; Singh, D.K.; Hasan, S.H.: Effective removal of lead ions using graphene oxide-MgO nanohybrid from aqueous solution: isotherm, kinetic and thermodynamic modeling of adsorption. J. Environ. Chem. Eng. 5(3), 2259–2273 (2017)
Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X.: Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 368(368), 540–546 (2012)
Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S.: Adsorption properties of tetracycline onto graphene oxide: equilibrium, kinetic and thermodynamic studies. PLoS ONE 8(11), e79254–e79254 (2013)
Pavagadhi, S.; Ai, L.L.T.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R.: Removal of microcystin-LR and microcystin-RR by graphene oxide: adsorption and kinetic experiments. Water Res 47(13), 4621–4629 (2013)
Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L.: Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohyd. Polym. 95(1), 501–507 (2013)
Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.; Cheng, R.: Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 268, 191–198 (2014)
Mohan, S.; Kumar, V.; Singh, D.K.; Hasan, S.H.: Synthesis and characterization of \(\text{ rGO/ZrO }_{2}\) nanocomposite for enhanced removal of fluoride from water: kinetics, isotherm, and thermodynamic modeling and its adsorption mechanism. RSC Adv. 6(90), 87523–87538 (2016)
Gong, Z.; Li, S.; Han, W.; Wang, J.; Ma, J.; Zhang, X.: Recyclable graphene oxide grafted with poly(N-isopropylacrylamide) and its enhanced selective adsorption for phenols. Appl. Surf. Sci. 362, 459–468 (2015)
Wang, X.; Huang, S.; Zhu, L.; Tian, X.; Li, S.; Tang, H.: Correlation between the adsorption ability and reduction degree of graphene oxide and tuning of adsorption of phenolic compounds. Carbon 69(69), 101–112 (2014)
Sakulpaisan, S.; Vongsetskul, T.; Reamouppaturm, S.; Luangkachao, J.; Tantirungrotechai, J.; Tangboriboonrat, P.: Titania-functionalized graphene oxide for an efficient adsorptive removal of phosphate ions. J. Environ. Manag. 167, 99–104 (2016)
Zong, E.; Wei, D.; Wan, H.; Zheng, S.; Xu, Z.; Zhu, D.: Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem. Eng. J. 221(221), 193–203 (2013)
Kovtyukhova, I.K.; Ollivier, J.O.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D.: Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 11(3), 771–778 (1999)
Kahani, S.A.; Jafari, M.: A new method for preparation of magnetite from iron oxyhydroxide or iron oxide and ferrous salt in aqueous solution. J. Magn. Magn. Mater. 321(321), 1951–1954 (2009)
Hu, X.; Yu, J.C.; Gong, J.; Li, Q.; Li, G.: \(\alpha \text{-Fe }_{2}\text{ O }_{3}\) nanorings prepared by a microwave-assisted hydrothermal process and their sensing properties. Adv. Mater. 19(17), 2324–2329 (2007)
Benjamin, M.M.; Leckie, J.O.: Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Colloid Interface Sci. 79(1), 209–221 (1981)
Lu, J.; Liu, H.; Liu, R.; Xu, Z.; Sun, L.; Qu, J.: Adsorptive removal of phosphate by a nanostructured Fe–Al–Mn trimetal oxide adsorbent. Powder Technol. 233(1), 146–154 (2013)
Wang, W.; Zhang, H.; Zhang, L.; Wan, H.; Zheng, S.; Xu, Z.: Adsorptive removal of phosphate by magnetic \(\text{ Fe }_{3}\text{ O }_{4} @\text{ C }@\text{ ZrO }_{2}\). Colloid. Surface. A. 469, 100–106 (2015)
Zhao, T.; Feng, T.: Application of Modified Chitosan Microspheres for Nitrate and Phosphate Adsorption from Aqueous Solution. RSC Adv. 6(93), 90878–90886 (2016)
Pan, M.; Lin, X.; Xie, J.; Huang, X.: Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. ASC Adv. 7(8), 4492–4500 (2017)
Vasudevan, S.; Lakshmi, J.: The adsorption of phosphate by graphene from aqueous solution. RSC Adv. 2(12), 5234–5242 (2012)
Han, C.; Wang, Z.; Yang, W.; Wu, Q.; Yang, H.; Xue, X.: Effects of pH on phosphorus removal capacities of basic oxygen furnace slag. Ecol. Eng. 89, 1–6 (2016)
Hui, B.; Zhang, Y.; Ye, L.: Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. Chem. Eng. J. 235, 207–214 (2014)
Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K.: Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 47(14), 5018–5026 (2013)
Wang, Z.; Shen, D.; Shen, F.; Li, T.: Phosphate adsorption on lanthanum loaded biochar. Chemosphere 150, 1 (2016)
Acknowledgements
This work is supported by the College students’ innovative experimental project of China (201610699222).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bai, L., Yuan, L., Ji, Y. et al. Effective Removal of Phosphate from Aqueous by Graphene Oxide Decorated with \(\varvec{\upalpha }\text {-}\hbox {Fe}_{2}\hbox {O}_{3}\): Kinetic, Isotherm, Thermodynamic and Mechanism Study. Arab J Sci Eng 43, 3611–3620 (2018). https://doi.org/10.1007/s13369-018-3124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-018-3124-3