Abstract
Fossil material from 90 fossil localities, mostly paleokarstic, has been gathered together to study western European bat evolution and diversity from the middle Eocene (~44 Ma) to the early late Oligocene (~29 Ma). The morphological and biometric observations and comparisons of the tooth material allow recognition of 7 families, 10 genera and 57 species; amongst the latter, 45 are described in detail here together with 11 subspecies. Several taxa of various systematic ranks are described as new: 1 subgenus and 17 species including 6 new subspecies; 1 new family and 2 new genera were described in a previously published paper but originate from this large review. From these new results, and the long period covered (more than 10 Ma), this work suggests a number of phyletic hypotheses. Amongst others, the relationship between the new fossil family Mixopterygidae and the fossil and extant families Emballonuridae and Hipposideridae is discussed. The peculiar Necromantis fossil genus being now better documented, its particular inferior molar pattern is exemplified as defining the necromantodont pattern. Even though the affinities of Necromantis remain unclear, the new data indicate that the previous assignment to Megadermatidae was incorrect. Thanks to the available information from the bat material, the relative dating of yet unstudied and undated new localities is proposed from biochronal reference stages, characterized by some bat species with a given size and morphology. Also, further data are given for faunas dated by the numerical ages method. The comparison of fossil and modern bat cenograms suggests that the body weight composition of a community is linked to the nature of the environment in which it evolves. Finally, these analyses allow deducing some possible bat evolutionary modalities either by the variations in the represented weight range or by the proportion of the different weight categories. They indicate that extinctions preferentially affected “extreme species” from a morphological point of view, as well as in terms of body mass. Consequently, this allows discussion of the effect of Stehlin’s faunal Grande Coupure faunal event amongst western European bats at the Eocene–Oligocene boundary.
Similar content being viewed by others
Introduction
In the “middle Eocene” or the Lutetian (including the Lutetian [47.8–41.3 Myr] and Bartonian [41.3–38.0 Myr] stages, Gradstein et al. 2012) and Bartonian (~41.3–38.0 Ma) stages, a humid, tropical climate prevailed in Europe. Land-based connections to the north of the developing Atlantic Ocean had been interrupted around 53 Ma ago. The western Holarctic mammal fauna of the lower Eocene grew more isolated from the other continental regions to the west (North America), the east and the south. It evolved much more endemically (Legendre and Hartenberger 1992), being characterized by the coexistence within the different mammal groups of relictual taxa and new species originating from regional evolution. Studies of several fossil groups show a general tendency to decreasing diversity in the last few million years of the upper Eocene, whilst at the same time new species were appearing. The extinctions, most likely driven by environmental changes such as decreasing temperatures, drought or opening environments, were accompanied by localized immigrations. This initial episode was called the Grande Coupure by Stehlin, the first to note its importance in 1909, and it is clearly observable in the rodent assemblages as well as at the entire terrestrial mammal community level (Legendre 1989; Escarguel and Legendre 2006; Escarguel et al. 2008). A significant phase of faunal renewal followed, starting at the very beginning of the Oligocene and lasting for several million years. This was a consequence of new continental passages being established to the north east of Europe with the closing of the Turgaï Strait, thus boosting faunal exchanges amongst Europe, Asia and America.
Many deposits from this transitional period have been found and exploited in the Quercy region of southwestern France. These lend temporal continuity and quality to the fossil record (Remy et al. 1987; Sigé and Legendre 1997). Not only do they provide information on the chronology of local populations but the significant amount of available material also allows a biometric approach. Previous studies of other mammal groups (e.g., rodents, artiodactyla, marsupials) have helped evaluate their diversity and thus to understand the evolution of the different lineages that came about with the changes towards the end of the Eocene. The karstic nature of the relevant deposits is particularly favourable for the presence and abundance of bats, even if it only provides an incomplete picture of their real diversity with a lack of data concerning cave-dwelling species. However, based on extant fauna Brosset (1966) stated: “Subterranean cavities are specifically home to a wide number of bats. Most of the families and genera include commonly or occasionally troglophile species, in tropical regions as well as temperate regions. In tropical countries, caves are generally a stable environment.”
Previous studies on the European middle Palaeogene have helped to define the presence of four main, large families of Chiroptera (Hipposideridae, Emballonuridae, Molossidae and Vespertilionidae) together with a few rare taxa, some with as yet undetermined affinities (e.g. Remy et al. 1987; Sigé 1988, 1995). All of these families have branched into today’s natural world (mostly the tropical Old World), and for a large part, examples of the Quercy genera can still be found. Pertaining to Chiroptera of the “classic” period of the Quercy (i.e. upper Eocene and Oligocene), descriptive studies have recorded a few specific moments of the chronological continuum in the form of collective monographs. These works have resituated several of the taxa described, based on specimens with wrongly attributed geographical and chronological origins and without a population-based context. They pertain to the Bartonian upper Eocene (Bretou fauna, MP 16 European Reference Level), the upper Eocene (Ludian as described by those authors; MP 18, Ste-Néboule fauna), and the late lower Oligocene (MP 25, Le Garouillas faunas and other chronologically close sites) (Sigé 1978, 1988, 1995, respectively). These initial results give the impression that the evolution of Chiroptera is notable in their dental remains, contrary to the common assumption of morphological conservatism in this group. The fossil diversity of these mammals has only been partially documented and their relationships are still disputed at the family level. The evolutionary characteristics of the group, its biochronology and its relationship with the environment can only be properly understood with a global study of available material, representing a long period of time.
Having access to a large quantity of specimens from European bat-bearing deposits, mainly karstic in nature and ranging from the middle Eocene to the upper Oligocene, opens the possibility of discovering essential information that had been missing until now. Their taxonomic biodiversity has now been recorded over a continuous period of almost 15 million years through the observation and comparison of dental material (not excluding the inclusion of osteology). Some revised phyletic lineages show relationships of direct succession and kinship between the different local populations and morphological bat species. Their appearance/disappearance over time corresponds to the influence of environmental modifications, notably those involved in the Grande Coupure. The long period of time covered, the observation of the increase in size of the species over time and the identification of reference stages based on morphology and size all serve to propose relative ages for localities as yet unexplored or still poorly studied, as well as to challenge the age of localities already dated by metric methods (Legendre and Bachelet 1993; Escarguel et al. 1997). Different criteria of morphological diversity (flight, echolocation, lifestyle, weight, dental morphology) are available through the biology and species ecology of extant taxa phylogenetically related to the fossils studied. The size of teeth, making analysis possible based on the fossil materials through the imputation of missing values, helps to further develop this point. Main component analysis of the shape of the tooth and the elaboration of cenograms from the body mass estimated for each species are used here to decipher the structure of a local or regional fauna and its evolutionary dynamics and modalities.
History of the Quercy Phosphorites
As most of the specimens in this study come from the Quercy Phosphorites, it is important to highlight the context in which they were discovered as well as the particular and favourable character of the fossiliferous filling in this region of southwestern France, which extends west to the Massif Central. The first phosphate mining operations began in the Quercy in 1870. As a natural fertilizer, it was widely used in agriculture and, as such, exported to England. The exploitation of Quercy phosphate reached its height in 1886 but the discovery of phosphate concentrations in the Somme appears to have led to its final decline in 1907 (Durand-Delga 2006). During those four decades, phosphate was not the phosphorites’ only claim to fame. The presence in the phosphate-laden clay of many well preserved, sometimes even exceptionally well preserved, fossil bones and teeth caught the attention of members of the scientific community, some of whom precociously reported on certain specimens (Daubrée 1871; Delfortrie 1872). These fossils, from various, often undisclosed, locations, were then sold not only to museum collections or universities, in France or abroad, but also to private collectors. These constitute what are today referred to as the “Old Quercy Collections”. The numerous discoveries nevertheless allowed many palaeontological studies (notably the many works of Filhol (e.g. 1872, 1876), Schlosser 1887 or Weithofer 1887). The first geologist to present precise observations about the phosphate pockets, their origins and the nature of their contents was Thévenin (1903). They had long been considered to be heterogeneous in age. Some scientists, Thévenin amongst them, had not hesitated in suggesting that this was merely due to the amalgamation of the Old Collections and that the Phosphorites’ fillings should no doubt reveal clear stratigraphies.
The interruption of the mining operations and the availability of relatively complete specimens led palaeontologists to study previously acquired collections. This lasted well into the first half of the twentieth century, with various works on materials coming from the mining operations, notably those by Teilhard de Chardin (carnivores (1914–1915), primates (1921)), and Revilliod about Chiroptera (1917–1922). Throughout this period, only the work of Gèze (1938) focuses on the geological and palaeontological particularities of the phosphate pockets and provides proof of the sedimentary and chronological homogeneity of the contents in situ. It was only from the 1960s that teams of French palaeontologists (Montpellier University, Paris Museum and Paris University) organized the field researches and excavations so as to pinpoint more precisely the locations of the material found. These were subsequently homogenous in both sediment and chronology and potentially datable through the study of the fauna that was included. In parallel with the palaeontological studies of the researchers involved on the large systematic groupings, other works were devoted to sedimentology, magnetostratigraphy and palaeoecology as well as the chronological interest with the synthesis and faunal and/or chronological updates of the deposits (Sigé et al. 1979; Crochet et al. 1981; Remy et al. 1987; Legendre et al. 1997; Sigé and Legendre 1997; Le Gall 2001; Astruc et al. 2003; de Franceschi et al. 2006; Maitre et al. 2006a; Sigé and Hugueney 2006 amongst others). The chronological study of the faunas in the region strongly contributed to the elaboration of the biochronological scale of mammals recognized and used by the community at large for European Palaeogene deposits (Schmidt-Kittler 1987; BiochroM’ 1997).
At the same time, focus was brought upon other Palaeogene palaeokarstic data from outside the Quercy (phosphorites from the Gard: Remy et al. 1997; neogene karstic deposits from the Bas-Languedoc and Roussillon: Aguilar and Michaux 1997; Sigé et al. 1997; Aguilar 2002; karstic deposits from the Swiss Jura, notably Mormont: Hooker and Wiedmann 2000). The financing, for a large part from the CNRS, is what made these works possible, with a large quantity of sediments collected and processed. Many studies for thesis projects and publications followed, notably in the 1970s (Hartenberger 1971; Vianey-Liaud 1971; Sigé 1974a, b; Sudre 1977; Crochet 1978; Lange-Badré 1979). This favourable period lasted until the 1990s (Godinot 1983; Legendre 1988; Cirot 1992; Augé 2001) when collective fieldwork and financing became more restricted. Prospecting and punctual collecting continued thanks more or less to individual initiatives, in a profitable collaboration with a highly motivated speleological team. Research topics on the materials available were put conducted at the universities (C. Blondel, B. Conte, F. Laudet in Montpellier, Y. Billaud, C. Le Gall in Lyon, E. Cirot, S. Peigné in Poitiers). These contributed to a better understanding of the Quercy, where Tertiary fossil deposits in situ are accounted for from the lower Eocene to the beginning of the Miocene and now the Pliocene (Aguilar et al. 2007). This work fits into a thematic continuity of research, proving that 140 years after their discovery, the Quercy Phosphorites remain a unique source of unexpected new data covering a time frame that has yet to be equalled in the rest of the world.
Material
The material studied comes from 88 French fossil localities, most of these in the Quercy region, and two Swiss localities (Fig. 1). Except for the lacustrine deposit of Aumelas, all are fillings of karstic networks within limestone terrains, dating from the Jurassic in the Quercy and the Cretaceous in the Bas-Languedoc region or the Swiss Jura, that were bored by water after a general decrease in sea levels (Astruc 1988). The alteration process generally remained active over several million years; in the Quercy, the estimated timeframe is 30 Ma starting from the lower Eocene. Erosion and kaolin-producing ferralitic alteration, aided moreover by the humid tropical Eocene climate, favour these plateaux due to the pre-existing structures (joints, joint networks and fault lines) and have created vertical crevasses and enlarged joints. These are plugged with clay and phosphate-laden sand fillings with phosphorite layers and concretions. The karsts became mostly open towards the surface and thus trapped materials such as sediments, plants or animal remain. These fillings are relatively complex and do not last very long. They may be fossiliferous and the grain more or less visible (Astruc 1988; Astruc et al. 2003). The sediments are generally loose, clay-like and more or less rich in residual alteration materials (e.g. ferruginous pisolites) or crystallized and endokarstic, phosphate-laden concretions (hence the name “Phosphorites du Quercy”) and calcitic cement (Billaud 1982). In some deposits, such as Perrière, Baraval or St Maximin, the alternation of colours which varies from reddish brown to white shows a clear stratification of the filling. The fossil material was extracted through screen washing the dried sediment samples. Blocks of well-dried clay are put into water to fall apart, thus freeing the fossils from the clay matrix. The resulting mud is then passed through screens of different sized meshes so as to separate the larger fossils from the rest. In general, the smallest size of mesh used is 0.5 mm as there are very few smaller specimens. The fossils collected are more or less fragmented and are sorted according to the systematic groups to which they belong. The fossils found are mainly mammal bones and teeth, and also remains of gastropods, urodela and frogs, squamata, testudines, and more rarely, plants (notably at Baraval and Monteils; de Franceschi et al. 2006; Maitre et al. 2006a). As for the specimens reviewed in this study, most of the material was already prepared for examination. Various new localities or additional collections in known localities were subjected to this relatively long and delicate screening process. Teeth constitute the majority of the remains as they are the most resistant elements in a body. They are generally well preserved, and more or less isolated depending on locality or even taxon (their habitat being more or less remote from the sedimentary location). All of the dental categories in the bat dentition can be found, ranging from incisors 0 to 3, of very poor quality due to their small size, to the 3rd molars. In this work almost 20,000 teeth were examined and measured [canines (C), premolars (P) and molars (M)]. These are small specimens (between 0.5 and 3 mm), generally referred to as microremains (Appendix 1). The bone remains studied are much less numerous and correspond to mandibles, although these are rarely complete, to extremities of the humerus, and to other elements of the skeleton.
A fraction of the fossils examined in this work, notably the types of classic taxa or some specimens attributed to the Necromantis genus, comes from collections dating back to the period when phosphate minerals were being mined in the last quarter of the nineteenth century. These collections were called the “Old Quercy Collections” and are generally notable for only having the term “Quercy” as an indication of their provenance, sometimes with more or less correct details about a village name close to the discovery site. In point of fact, these specimens, having been found and often sold by the workers, were spread across many different museum and university collections, not to mention private collections. Even today, they are usually not tied to a locality and are therefore of indeterminate age. For the most part, the specimens from the Old Collections used in this work come not only from the Naturhistorisches Museum in Basel, but also from the Naturhistorisches Museum in Vienna, the “Centre de Collection et de Conservation” of Lyon 1 University, the Muséum d’Histoire Naturelle in Montauban, and the Muséum d’Histoire Naturelle in Paris.
However, new excavations carried out as early as the 1960s by various French universities, Université Montpellier 2 first amongst them, helped and continue to help reveal a large amount of fauna in the Quercy region (Maitre et al. 2006a; Sigé and Hugueney 2006). They also help to obtain precisely located specimens, which can therefore be dated. It is from these that come most of the materials used in this work. They are housed in the collections of Université Montpellier 2. To make use of the various systematic works based on specimens from the Old Collections, Sigé (1978) proposed to revalidate the classic Chiroptera taxa by assigning reference populations from known localities and thus make it possible to date them. These populations are chosen for how well they correspond in morphology and size to the older named material. This has the advantage of providing more information of a systematic nature due to the better material records, in an evolutionary context, and a precise chronological reference framework. The 90 localities from which the materials were taken are spread from the upper Lutetian (middle Eocene, ~44 Ma) to the Chattian (upper Oligocene, ~29 Ma) (see Appendix 2). Based on their faunal contents, most of them are biochronologically related to a reference level of the biochronological scale for European mammals as defined in the symposia of Mayence (Schmidt-Kittler 1987) and Montpellier (BiochroM’ 1997). Once there is enough material from a certain locality, it is associated to a numerical age (Legendre and Bachelet 1993; Escarguel et al. 1997). The interval between the studied reference levels ranges from MP 13 to MP 25. Faunas at reference levels MP 23 and 24 do not represent all of the known faunas from these biochronological units. They are taken into account because Gardiol 3, the subject of my Master 1 research dissertation, and Lébratières 14, a small fauna with hitherto unstudied Chiroptera, help to make the transition to the upper Oligocene localities of reference level MP 25, Le Garouillas and other contemporary sites, for which a study of faunal contents has already been mostly performed during a recent monograph (Sigé 1995). This study proposes a relative age for several localities where the faunal content has yet to be studied (either recently discovered or almost entirely composed of Chiroptera, e.g. Liauzu).
Methods
The dental nomenclature used in this paper is derived from van Valen (1966) and Szalay (1969), shown in Fig. 2a. The nomenclature used for the description of the mandible is shown in Fig. 2b.
Reference numbers
Each new specimen examined in this study is referenced by a number that includes an acronym for its locality followed by the initials of the species and finally an inventory number. The collection to which these specimens belong is also shown in the measurement tables. All the abbreviations used for the names of localities are given in Appendix 3. Institutional abbreviations: Naturhistorisches Museum Basel and Wien (QP, QH); Muséum National d’Histoire Naturelle de Paris (MNHN); Centre de Collection et de Conservation (C3GL); Université Claude Bernard, Lyon1 (UCBL1); Collections de l’Université Montpellier 2 (UM2); Muséum d’Histoire Naturelle de Montauban (MnCh1).
Measurements
Descriptions and comparisons of all the material in this study are based on observations made with a stereo microscope. Measurements are taken on the teeth that are most representative of the morphology of this group: canines, premolars (only if these are not vestigial) and molars. Measurements have been taken using a NIKON MM-40 measuring microscope equipped with a QUADRA-CHEK 200 calculator and a 30× lens; they are listed in Fig. 3.
Dental morphology
Chiroptera possess several different structural types of lower molars. To simplify their identification during the description of dental material, various authors took part in their characterization and denomination. Thus, it seems appropriate to evoke the varied morphologies that are already well known. Two different types were introduced by Menu and Sigé (1971), based on the relative positions and existing connections between the three cusps of the talonid (entoconid, hypoconid and hypoconulid). Nyctalodonty, from the genus name Nyctalus (Fig. 4a), occurs when the hypoconulid is well developed and directly connected to the hypoconid by the postcristid. This structure is found in all known families since the appearance of chiroptera in the fossil record, at the very beginning of the Eocene, and in many of the genera studied here, notably Hipposideros (Pseudorhinolophus) or Vaylatsia (both hipposideridae). Myotodonty (a structure clearly displayed by the genus Myotis; Fig. 4b) occurs in a talonid where the hypoconid is directly connected to the entoconid by the postcristid, isolating the hypoconulid, and usually of small size. This structure is frequently found in Vespertilionidae and Noctilionidae and can be observed in a small number of individuals within some species of the genus Vespertiliavus or Cuvierimops (Emballonuridae and Molossidae, respectively). These two configurations are the most commonly observed amongst chiroptera. Myotodonty appears later in the fossil record, near the end of the lower Eocene. An intermediate configuration somewhere between these two extremes, called submyotodonty, was suggested by Legendre (1984b) based on Molossidae material. This usually only appears in a few specimens of a population that remains for the most part myotodont in nature. It is characterized by an entoconid and a hypoconulid, only slightly reduced, both connected to the hypoconid by the postcristid. This work has helped to characterize another type of structure, necromantodonty (Sigé et al. 2012), generally seen in lineages from the lower Eocene and then subsequently lost (Sigé et al. 2007). The hypoconulid is thus in a median position between the entoconid and the hypoconid to which it is connected. It can be very pronounced (Fig. 4c). Examples of this are the genera Necromantis, Palaeophyllophora, Honrovits, Ageina or Australonycteris. Necromantodonty and nyctalodonty occurring from the very beginning of the Eocene suggest that these two structural types already coexisted within Palaeocene Chiroptera, which nevertheless still remain to be discovered.
Systematic Palaeontology
Order Chiroptera BLUMENBACH, 1779
Sub-order Microchiroptera DOBSON, 1875
Superfamily Vespertilionoidea GRAY, 1821 (WEBER, 1828)
Family Vespertilionidae GRAY, 1821
“Members of this family are recognizable externally by their simple muzzles and lips, usually separate ears with well-developed, straight, or slightly curved tragi, long tails extending to edge of wide interfemoral membrane, but never beyond; presence of only two bony phalanges in third finger, and absence of sucking disks on sole and thumb. Internally, they are distinguished by the highly developed double articulation between scapula and humerus, the very rudimentary ulna, the essentially unmodified shoulder girdle and pelvis” (Miller 1907). Menu (1987) completes this definition: “All Vespertilionidae have the following characters in common:
-
premaxillaries fused to other bones in the cranium;
-
no fusion of the ischium;
-
rudimentary and practically non-functional fibula;
-
double-contact articulation between the humerus and scapula;
-
no fusion of the 7th cervical vertebra with the 1st dorsal vertebra (except in Tomopeatinae);
-
advanced adaptation for flight, without reaching the remarkable possibilities of the neighbouring Molossidae family;
-
despite already existing morphological variability and undeniable emerging evolutionary trends, there are various un-specialized tooth characters suggesting possible morphological evolution can occur.”
Current geographic distribution: both hemispheres up to the limit latitude for tree growth; the Azores (Atlantic), the Galapagos Islands, the Hawaiian Islands, New Zealand and Samoa (Pacific).
Genus Leuconoe BOIE, 1830 (sensu Menu 1987, 1988)
Original diagnosis: that of Boie, to be revised with additional information pertaining to morphology and variability from Menu (1987, 1988)
Type-species: Leuconoe salodorensis (Boie, 1830)
Other species described: Leuconoe lavocati (Boie, 1830)
Distribution: Oligocene in western Europe (MP 22–26).
Leuconoe (Leuconoe) sp. indet. A (Fig. 5; Plate 1a)
Leuconoe (Leuconoe) sp. indet. A from Baraval: a BALch_01, right hemimandible with alveoli for I/1–2 and P/4, and with I/3, C/1, P/2–3 and M/1–2–3. Cuvierimops parisiensis parisiensis (Cuvier in Pictet 1844) from Rosières 2: b ROS2_CuppaA.1.27, left C/1, labial view (left) and lingual view (right). c ROS2_CuppaA.2.6, fragment of right hemimandible with alveoli for I/1–2, C/1, P/2–4, and with M/1 and broken M/2. d ROS2_CuppaA.2.7, right M/1. e ROS2_CuppaA.2.24, fragment of left hemimandible with alveoli for C/1, M/1 and with P/3–4 and M/2–3. f ROS2_CuppaA.3.12, right P4/. g ROS2_CuppaA.3.4, right M1/. h ROS2_CuppaA.3.6, right M2/. i ROS2_CuppaA.3.15, right M3/
Synonymy: 1998: Fam., gen. and sp. indet. in Sigé et al., p. 87
Locality: Baraval (MP 22), Lot, Phosphorites du Quercy, France
Material and measurements: unique specimen, see Appendix 4
Description: Lower incisors: only the root of last remaining posterior incisor, adpressed to the base of the canine; anteriorly, a broken hemimandible, presumed existence of two other incisors from partial observation of an alveolus (more anterior and lingual than the root in question) and of a space anterior to the mandibular symphysis.
C/1 (at the broken extremity), with a pronounced surrounding ridge, raised at the front with a little anterior relief.
P/2–3 extremely compressed and small; P/3 smaller than P/2, both uniradiculate and with similar morphology; thick ridge all around, oval occlusal contour, presence of anterior and posterior lingual relief starting at the ridge.
P/4 (known only by alveoli) biradiculate, clearly larger than the other premolars.
M/1–3: myotodont, talonid composed of two massive cusps: the hypoconid and entoconid; less pronounced hypoconulid on the posterior side of the entoconid, clearly lingual on M/2; thick cingulids; crista obliqua connecting to the trigonid on the lingual side of centre; M/1 with narrower trigonid than M/2; M/3 with reduced talonid, the hypoconulid clearly separate.
Comparison: regarding the two fossil species of Leuconoe found at Le Garouillas site and contemporary localities of reference level MP 25, L. salodorensis and L. lavocati, the Baraval specimen is closer to the first in size, even if the morphology of the molars shows clear similarities with the three taxa: a pronounced entoconid at the posterolingual corner of the tooth, an hypoconulid pitched towards the lingual edge, an M/3 with a hypoconulid, and a pronounced axial tilt of the crista obliqua. However, several characters separate them: the Baraval specimen has wider molars, with a less open trigonid on the lingual side, a smaller hypoconulid, a straighter, less lobed labial edge (with a clear separation between trigonid and talonid in L. salodorensis), a talonid clearly larger than the trigonid, and thicker cingulids. The premolar region is not represented in the material from level MP 25 but a comparison with the material of Leuconoe (L.) emarginatus, as extant, small species, shows the P/2–3 area (uniradiculate but pronounced) clearly less cramped in the extant specimen, whereas P/4 seems to have the same proportions when it comes to the size of the molars. The molars themselves are similar, although the hypoconulid is more cuspidate and developed, the cingulids are slightly narrower, and the talonid of M/3 slightly smaller on the extant specimen.
Finally, the Baraval specimen is closer to the extant genus Plecotus in the number of premolars, with P/4 larger than P/2–P/3 and the strong compression observable at this point. However, despite the myotodont molars in both taxa, those of the vespertilionid from Baraval are noticeably closer to those of Leuconoe. The lack of information related to premolars in the genus Leuconoe during this period, as well as to the upper molars of the Baraval taxon, results in a determination using open nomenclature, until new material is found.
Remarks about Leuconoe (Leuconoe) emarginatus: This extant vespertilionid species, previously attributed to the genus Myotis KAUP, 1829, is here assigned to the genus Leuconoe and the subgenus of the same name, based on the works of Menu (1987, 1988) on the dental morphotypes of extant and fossil Vespertilionidae. This author notes the great variability of the genus and the subjective distribution of the morphotypes into several subgenera. The failure of this classic classification to align with evolutionary observations on dental morphology led him to group into the subgenus Leuconoe the species classified in the literature in the subgenera Selysius, Isotus, Paramyotis and Rickettia.
General remarks on Vespertilionidae: This work did not bring to light any new elements pertaining to this species so similar to those of the genus Leuconoe. Some isolated specimens, found in the non-karstic upper Eocene of Le Batut locality (MP 19), east of the Quercy Phosphorites, are related to Vespertilionidae (Muratet et al. 1985 in Pl. 1, Fig. 8). However, the lack of data dealing with the lower tooth-rows prevents further comparison. Lower tooth-rows referable to this family have been found in Hoogbutsel (Lower Oligocene, Belgium, MP 21) and have been described by Quinet (1965) under the name Myotis misonnei. This taxon, since attributed to the new genus Quinetia by Horacek (2001), is characterized by the same shortened premolar row, but displays clear nyctalodonty in the lower molars.
The identification of several forms definitively belonging to Vespertilionidae as early as reference level MP 21 leads to the conclusion that this family was already widely diversified, indicating either that these bats appeared in the faunas of western Europe several reference levels earlier or that the centre of their diversification was outside Europe.
Family Molossidae GILL, 1872
“Humerus with trochiter much larger than trochin; the discrepancy in size usually more noticeable than in the Vespertilionidae; trochin articulating with scapula by a surface aspect nearly as large as glenoid fossa; epitrochlea short, but with very conspicuous spinous process; capitellum almost directly in line with nearly straight shaft; ulna less reduced than in Vespertilionidae, the very slender shaft usually about half as long as the radius; second finger with well-developed metacarpal and one rudimentary phalanx; third finger with three phalanges, of which the first is flexed on upper side of metacarpal when wing is at rest, and third is cartilaginous except occasionally at extreme base, where distinct joint is formed with middle phalanx; fifth finger scarcely longer than metacarpal of first; shoulder girdle normal (¼), except that seventh cervical vertebra is fused with first dorsal vertebra; foot short and broad, but of normal structure; fibula complete, bowed outward from tibia, its diameter about half that of the latter, entering conspicuously into the mechanical scheme of the short, stout leg” (Miller 1907).
Current geographic distribution: Warmer portions of both hemispheres; from the North of the Old World to southern Europe and southern Asia, east to New Guinea, Australia and Norfolk Island; in northern America to the southern states and throughout the West Indies.
Genus Cuvierimops LEGENDRE & SIGÉ, 1982
Dental formula: I 1?/2, C1/1, P2?/2, M3/3
Original diagnosis: same as type-species
Type-species: C. parisiensis parisiensis Cuvier (in Pictet 1844)
Other species described: C. parisiensis priscus nov. ssp.; C. parisiensis intermedius nov. ssp.;
C. legendrei nov. sp.
Distribution: from the upper Eocene (MP 17a) to the upper basal Oligocene (MP 25) of western Europe (France).
Cuvierimops parisiensis CUVIER (in Pictet 1844)
Remarks: Cuvierimops parisiensis is found in the upper Eocene Quercy fillings (from reference levels MP 17a to MP 19). It remains relatively consistent in its general morphology during this period. Despite it spanning only a few million years, the material reveals evolution of the structural type of the lower molars. Thus, within a general morphological context, in certain specimens the nyctalodont structural schema becomes submyotodont or even myotodont. This change seems to be accompanied by a notable increase in general size. Based on these minor differences, it is possible to separate the evolutionary stages into three successive subspecies along time: C. parisiensis priscus (MP 17a), C. parisiensis intermedius (MP 17b and MP 18) and C. parisiensis parisiensis (MP 19).
Synonymy: see Legendre & Sigé, 1982
1973: cf. Tadarida sp. 1, sp. 2 in de Bonis et al., tabl. 2a
1981: cf. Tadarida sp. in Crochet et al., tabl. 2-2
1984a: Cuvierimops parisiensis in Legendre, Pl. 1
1985: Cuvierimops parisiensis in Legendre, p. 208–209, fig. 16 p. 220
1985: Cuvierimops parisiensis in Sigé, p. 182.
Diagnosis: same as C. parisiensis parisiensis.
Cuvierimops parisiensis parisiensis (CUVIER in Pictet 1844) (Plate 1b–i)
Synonymy: 1987: Cuvierimops sp. in Remy et al., tabl. 2a, p. 180.
Original diagnosis: the largest species of Cuvierimops observed in the Quercy faunas. Amended diagnosis (Legendre and Sigé 1982): small size; high coronoid apophysis (height exceeding M/1–M/3 length); nyctalodont lower molars, lower premolars biradiculate; P/4 with rudimentary metaconid; reduced lower canine; M1/ and M2/ with isolated conical hypocone; M3/ less reduced with metacone; pronounced epitrochlea, and styloid process clearly separated from the anterior edge of the trochlea.
Amended diagnosis: lower molars mostly nyctalodont, and sometimes myotodont or submyotodont; M1/ and M2/ with a conical hypocone.
Derivatio nominis: nominal subspecies. Holotype (monotype): partial skeleton on gypsum plate, without inventory number, from the collections of the Museum National d’Histoire Naturelle (Paris), fig. 1–3’, Pl. 1 in Legendre & Sigé (o.c.).
Type-locality: Montmartre (MP 19), Paris Basin, France.
Other localities: Rosières 1, Rosières 2, Escamps, Célarié ocre, Célarié standard (MP 19).
Material and measurements: see Appendix 4.
Description: the material recorded here completes that of Montmartre and provides more nuances and details to the commentaries by Legendre and Sigé (1982). The following morphological descriptions characterize all subspecies of C. parisiensis. C/1: not very tall, with pronounced lingual ridge ending both anteriorly and posteriorly in a small relief, directed more posteriorly; slight labial cingulum; posterior aspect almost flat, anterior aspect convex.
P/2 (observed for C. parisiensis parisiensis and C. p. intermedius nov. ssp.): biradiculate, relatively oblique on the hemimandible with the anterior root most labial; thicker ridge on lingual side, developing a small anterior and posterior relief (inclined backwards); smaller than P/4; monocuspidate; internal aspect flat, external aspect convex.
P/4 biradiculate, but angle of roots more moderate than on P/2; formed by a trigonid with a dominant protoconid, from which originate two crests (one anterior and one posterior), ending on the lingual side with a small relief raised in comparison to the cingulid; well-developed cingulid around the entire tooth, extended and cuspidate at its posterolingual end; thick and wide ridge, mostly on the labial and posterior side.
Lower molars with narrow trigonid, bearing a pronounced entoconid and a small hypoconulid inclined backwards; structural schema of M/1–2 presenting intermediate stages between: the major nyctalodont type with hypoconulid more or less distant from entoconid; the submyotodont type for C. parisiensis intermedius nov. ssp. (4 % in Perrière sample, 6 % in Malpérié); and the myotodont type (up to 6 %) for C. parisiensis parisiensis (Fig. 6); long entocristid; ante- and postcingulids larger than the labial cingulid, indented between the trigonid and talonid; deep talonid behind the metaconid; trigonid of M/1 more open and narrower than on M/2.
M/3 of C. parisiensis parisiensis and C. p. intermedius nov. ssp. with less reduced talonid, bearing a hypoconulid.
C1/: well-developed ridge around margin; convex labial aspect, delimited by clean edges, and composed of a central area of relief flanked by two vertical, background grooves; flat lingual aspect; surrounding cingulum, thicker on the lingual side; marked variation in size within the Perrière population.
P2/: not insignificant uniradiculate stylet.
P4/: dominant paracone; protocone on the anterior edge with a preprotocrista rising towards the labial edge, and a postprotocrista bordering a slight talon and joining the postcingulum.
M1–2/: clearly separate paracone and metacone; parastyle present, highly curved towards the inside of the jaw; wide cingula with uninterrupted connection to the protocone; trigon with a paraloph (decreasing before the edge of the tooth), a metaloph (sometimes crossing the bottom of the protofossa towards the base of the paracone, one such example from Rosières 2), and a basin, most deep in the middle of the tooth, at the point of occlusal impact with the hypoconid; anterolingual cingulum at the base of the protocone; strongly cuspidate hypocone, connected to the protocone by a pre-hypocrista, sometimes lingually offset on M1/ and with a posterior ridge; crestiform mesostyle, rounded, located on labial edge, previously low-necked; M1/ distinguished from M2/ as narrower anteriorly; postcingulum bordering on the posterior part of talon, and rising on the side of the hypocone; cingulum at the base of the protocone. N.B.: molar LEB1 (CuppaA.1.6), more worn, with metaloph; and small, cuspidate anterior relief at the base of the protocone.
M3/ slightly smaller; metacone present; pronounced parastyle, clearly labial compared to the mesostyle; preprotocrista joining the parastyle and postprotocrista joining the base of the metacone.
Cuvierimops parisiensis priscus nov. ssp. (Plate 2a–f)
Cuvierimops parisiensis priscus nov. ssp. from Lébratières 1: a LEB1_CupprA.1.1, left C/1, labial view (left) and lingual view (right). b TRI_CupprA.1.1, left P/4. c ABL2_CupprA.1.1, right M/2. d LEB1_CupprA.1.5, right C1/. e LEB1_CupprA.1.9, right P4/. f LEB1_CupprA.1.7, holotype, right M1/. Cuvierimops parisiensis intermedius nov. ssp. from Perrière and Malpérié: g PRR_CupiI.2.1, left C/1, labial view (left) and lingual view (right). h PRR_CupiD.1.3, fragment of left hemimandible with alveoli for P/4 and with I/1–2, C/1, P/2. i PRR_CupiI.1.1, right C1/, labial view (left) and lingual view (right). j PRR_CupiE.1.3, fragment of left hemimandible with alveoli for C/1 and P/2 and with P/4 and M/1–3. k MPR_CupiA.3.2, right P4/. l PRR_CupiA.1.7, left M1/. m PRR_CupiB.1.1, holotype, fragment left maxillary with M2–3/
Synonymy: 1981: cf. Tadarida sp. in Crochet et al., tabl. 2-2
1987: Cuvierimops sp. in Remy et al., tabl. 1a, p. 177.
Diagnosis: subspecies smaller than C. parisiensis intermedius.
Derivatio nominis: from the Latin adjective priscus: first, as it is the oldest of the known subspecies to date.
Holotype: LEB1_CupprA.1.7, left M1/ (Plate 2f), from the collections of UM2.
Type-locality: Lébratières 1 (MP 17a), Lot, Phosphorites du Quercy, France. Other localities: La Bouffie, Aubrelong 2, Trifon (MP 17a).
Material and measurements: see Appendix 4.
Description: morphology of dental categories identical to those of other subspecies of C. parisiensis.
Cuvierimops parisiensis intermedius nov. ssp. (Plate 2g–m)
Synonymy: 1981: cf. Tadarida sp. in Crochet et al., tabl. 2-2
1987: Cuvierimops sp. in Remy et al., tabl. 1a p. 177
2006a: Cuvierimops sp. A in Maitre et al., p. 118, fig. 5a
Diagnosis: Species of intermediate size between C. parisiensis priscus and C. parisiensis parisiensis.
Derivatio nominis: from the Latin adjective intermedius: in the middle of, intermediary, due to its size being intermediate between the two other known subspecies.
Holotype: PRRCupiB.1.1, left M2–3/ (Plate 2m), from the collections of UM2.
Type-locality: Perrière (MP 17b), Tarn-et-Garonne, Quercy Phosphorites, France. Other localities: Malpérié, Coyrou 3, Sorcières (MP 17b), Théron, Crégols (MP 18). Material and measurements: see Appendix 4.
Description: morphological characteristics identical to those of the other subspecies of C. parisiensis, but providing additional information about the lower incisors: observation of some variability in number; in two cases specimens with two incisors, quite pronounced and of same size; and specimens with three incisors, including a vestigial I/3. Comparison of C. parisiensis to other species of the same genus:
C. legendrei nov. sp. (see below) is larger than C. parisiensis parisiensis, and its morphology seems more advanced towards a myotodont dental pattern, and the simplification of the tooth row. C. sp. indet. A, a notably more recent species from Le Garouillas (MP 25), is equivalent in size to C. parisiensis priscus nov. ssp., but can be morphologically distinguished by the M1–2/ hypocone, being much more detached from the protocone, even if a slight crest sometimes connects them; the paraloph is less pronounced than the metaloph; the C1/ has a thicker ridge on the labial aspect, sinuous anteriorly, and a wide ridge on the lingual aspect, creating a significant horizontal shelf.
Cuvierimops legendrei nov. sp. (Plate 3)
Cuvierimops legendrei nov. sp. from Baraval: a BAL_CulC.3.4, right C/1, labial view (left) and lingual view (right). b BAL_CulB.3.2, fragment of left hemimandible with alveoli for I/1–2, C/1 and with P/2–4, M/1–2. c BAL_CulB.2.3, right M/1, submyotodont. d BAL_CulB.2.12, right M/1, myotodont. e BAL_CulB.1.5, left M/3. f BAL_CulC.1.5, right C1/ labial view (left) and lingual view (right). g BAL_CulA.3.19, left P4/. h BAL_CulA.1.10, right M1/. i BAL_CulA.2.4, holotype, left M2/. j BAL_CulA.2.18, left M3/
Synonymy: 1987: Cuvierimops sp. in Remy et al., tabl. 3a, p. 183
Diagnosis: large-sized Cuvierimops species; P/2 sometimes with single root monoradiculated, smaller than that of C. parisiensis; lower molars of variable structural form, with simultaneous presence of nyctalodont to myotodont morphologies (the latter being slightly more frequent in other species of the genus).
Derivatio nominis: In honour of Dr. Serge Legendre for his contribution to the systematics and phylogeny of Molossidae, notably the genus Cuvierimops.
Holotype: BAL_CulA.2.4, left M2/, (Plate 3i), from the collections of UM2.
Type-locality: Baraval (MP 22), Lot, Phosphorites du Quercy, France.
Other localities: Lébratières 13, Lébratières 15 (MP 22), Gardiol 3 (MP 23).
Material and measurements: Appendix 4.
Description: C/1 practically endowed with a trigonid having an independent “paraconid” and both crests from the “protoconid” well defined; surrounding cingulid; slightly convex anterolabial aspect, both other faces slightly concave, with an extended margin at the posterior base, notably on the lingual side.
P/2 small, uni- or biradiculate depending on the specimen.
P/4 typical of the genus.
M/1–2 mostly nyctalodont (92 %) with hypoconulid more or less distant from the entoconid, as far as showing submyotodont morphology (4 %), or even myotodonty (4 %). Short C1/ lamella, completely surrounded by a distinct cingulum; flat lingual aspect, slightly convex in the middle of the lamella, and convex labial aspect.
M1–2/ typical of the genus, sometimes with labial and posterior cingulum. M1/ can display distinct, tall hypocone, strongly extended posteriorly, and blending into the ridge of the talonid; indentation between the paracone and metacone sometimes very pronounced.
M3/ typical of the species.
Comparison: the general morphology is still well preserved here and few characters, other than being larger in size than C. parisiensis, are distinctive. The myotodont morphology, a character state revealed to be “evolved” (from the observations made in this work), seems to still be under-represented in more conservative general morphology. However, this slowly involves a growing number of specimens. Furthermore, the simplification of the anterior part of the jaw seems to begin with progressive anteroposterior reduction and compression of P2 (Fig. 7). The C1/ morphology is identical to that of the other known Cuvierimops, but the size range is smaller than that of the Perrière canine. Cuvierimops sp. indet. A from Le Garouillas (MP 25) (Sigé 1995), a locality close in age to those where C. legendrei nov. sp. specimens were found, is notably smaller.
General discussion on the genus Cuvierimops: There have never been many available specimens for the various Cuvierimops species. The material always seems more fragile and damaged than the rest of the bat fauna, and complete tooth rows are rare. This could be due to longer post-mortem transport for this material compared to that of other genera. This leads to the hypothesis of these species being more likely to colonize cave entrances or their periphery, as opposed to hipposiderid or emballonurid species, which would have lived deeper in the cave system. Their remains, closer to the site of sedimentation, are noticeably better preserved.
The specimens from Lébratières 1, Perrière and Rosières 2 stand out from the other, relatively well-documented localities of this genus (Malperié, Baraval) by their intraspecific wide size range (cf. measurements, Appendix II.2). Despite this, the range of sizes appears to be homogenous. This qualitative observation is quantified with a Shapiro–Wilk test of normality. The test is applied to the lengths of M/1 and M1/ of a population with a narrower range of sizes (Baraval). The probabilities calculated in both cases are greater than 0.05. Thus, the hypothesis of normal homogeneity of the material in each of the populations cannot be rejected (Tableau II.2.1). The standard deviation highlights the reality of a broader range of sizes at Perrière than at Baraval. Nevertheless, the mixture analysis of the specimens from Perrière (from which two specimens were excluded due to their significantly smaller size) does not evidence strong difference between unimodal distribution (100 %: μ = 1.78 ± 0.061; AIC = −213.4) and bimodal distribution (13.7 %: μ 1 = 1.85 ± 0.014 and 86.3 %: μ 2 = 1.77 ± 0.060; AIC = −211.8) (Table 1).
These results suggest a size-related dimorphism. This phenomenon is regularly observed in several genera of extant and fossil bats, including Molossidae (Revilliod 1917–1922; Engesser 1972; Ziegler 1993; Sigé et al. 1997). It is often linked to the sex of the individuals. Generally speaking, the main differences concern the size of the canines. These are considered to be a secondary sexual characteristic, found here in relation to the rest of the dentition, notably the P/2 (Legendre 1982). With such large gaps in fossil size (one sex clearly bigger than other), these three localities present a mixing of the sexes that points to an unsegregated lifestyle in the diurnal roost, at least at one point of the year. Other sites record either seasonal segregation or a segregation confined to the roost. This is a relatively well-known phenomenon amongst extant Chiroptera, particularly in most Vespertilionidae. This sexual dimorphism has already been observed in fossil populations of Molossidae (Tadarida), in the canines and P/2 premolars, whereas there are no records of skeletal discrepancies. Elements that are small in size, generally connected to the females, are thus observed in greater quantities within the roost (Legendre 1982). This may explain the smaller size of the specimens in the homogenous deposits.
Family Palaeochiropterygidae REVILLIOD 1917
Amended diagnosis (Russell and Sigé 1970): low trochin and trochiter; no secondary articulation between humerus and scapula; phalangeal formula 2.1.2.2.2 for anterior limb; second finger without claw.; radius and fingers relatively longer than in Archaeonycteridae. Slender cuspids. Molariform P/4. Upper molars with normal ectoloph.
Genus Stehlinia REVILLIOD, 1919
Synonymy: 1922: Nycterobius in Revilliod, p. 133–136, fig. 47, Pl. 4, fig. 1–6
1922: Paleunycteris in Revilliod, p. 144–149, fig. 55–58
1945: Revilliodia in Simpson, p. 59
Amended diagnosis (Sigé 1974a): Vespertilionoid with generalized cranial and dental characters: full premaxillaries; dental formula 2/3 1/1 3/3 3/3; biradiculate or triradiculate P3/; P/3, biradiculate, as large as P/4; simple P4/ and P/4, non-molariform; upper molars without hypocone; nyctalodont lower molars, with large, wide and tall talonid; double articulation between scapula and humerus; evolved elbow, with narrow, non-spherical condyle. Type-species: Stehlinia gracilis (Revilliod, 1922), Old Quercy Collections (unknown locality and indeterminate age), France. Other species described: S. gracilis (gracilis, mutans nov. ssp.); S. minor; S. quercyi; S. pusilla; S. rutimeyeri; S. bonisi; S. revilliodi nov. sp.; S. alia nov. sp.; S. sp. A; S. sp. B.
Distribution: from the middle Eocene (MP 13) to the upper basal Oligocene (MP 25) in western Europe (France).
Stehlinia gracilis (REVILLIOD, 1919).
Remarks: the species named by Revilliod is observed from the upper Eocene (MP 17a) up to the lower Oligocene (MP 23). Despite the dental form remaining mostly the same throughout this time period, two morphological states can apparently be distinguished. The taxon is now separated into two subspecies. Diagnosis: same as S. gracilis gracilis.
Stehlinia gracilis gracilis (REVILLIOD 1922) (Plate 4)
Stehlinia gracilis gracilis (Revilliod 1922) de Malpérié: a MPR_SggA.2.28, left C/1, labial view (left) and lingual view (right). b MPR_SggA.1.12, fragment of right hemimandible with alveoli for C/1 et M/1 and with P/2–3–4. c MPR_SggA.1.2, fragment of right hemimandible with alveoli for P/3–4 et M/3 and with M/1–2. d MPR_SggA.1.14, left M/3. e MPR_SggC.1.4, right C1/, labial view (left) and lingual view (right). f MPR_SggA.3.9, fragment of left maxillary with P4–M3/. g MPR_SggA.4.22, right M3/
Synonymy: 1922: Nycterobius gracilis in Revilliod, p. 133–136, fig. 47, fig. 1–6, Pl. 4.
1979: Stehlinia gracilis in Sigé et al., p. 49
1981: Stehlinia gracilis in Crochet et al., tabl. 2-2
1987: Stehlinia gracilis in Remy et al., tabl. 1a p. 177
Amended diagnosis (Revilliod describes and compares the only specimen at his disposal, but does not strictly speaking provide a diagnosis. Due to the more substantial quantity of material presently available, the characteristics of this taxon can be more detailed in this study): species with narrow dentition, lower molars proportionally longer than those of S. minor, which also show a trigonid and talonid of subequal width; smaller size than S. minor.
Derivatio nominis: nominal subspecies.
Holotype (monotype): Q.P. 632, right maxillary fragment displaying alveolus for I2/, C1/, P23/ and bearing P4/–M1–2–3/, fig. 47; pl. 4, fig. 1–6, Revilliod (1922).
Type-locality: Old Quercy Collections (unknown locality, undetermined age), France.
Reference population: Malpérié (MP 17b), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Lébratières 1, Aubrelong 2, Trifon, Clapassou (MP 17a), Perrière, Coyrou 3 (MP 17b).
Material and measurements: see Appendix 4.
Description: the mandible has an ascending ramus quickly becoming vertical posterior to M/3, and an oblique base distinctly elevated in relation to the horizontal ramus; deep masseteric fossa, on the same oblique axis as the base; angular apophysis angled laterally; horizontal ramus height equivalent to one and a half than that of the molar crown; long, narrow condyle aligned with the tooth row but better developed medially; wide mental foramen, located beneath P/2, and open cranially (Fig. 8a).
C/1 almost as tall as C1/; surrounding cingulid, anteriorly and posteriorly cuspidate; principle cusp separated from two smaller ones by a sinus.
P/2 uniradiculate, with oval occlusal outline.
P/3–4 biradiculate; P/3 of subequal size to P/4; surrounding cingulid, undulating posteriorly and with a small relief at the anterolingual corner, connected by the preparacristid; more occlusally elongated anteriorly; postparacristid connected to lingual margin, sometimes slightly concave labially.
Nyctalodont lower molars with variable space between entoconid and hypoconulid; massive entoconid, with three aspects: one convex lingual surface and two flat (one anterolabial and the other posterolabial, delimited by sharp edges); relatively undeveloped hypoconulid, generally crestiform and extending posteriorly; trigonid of M/2 more open than that of M/1.
M/3 low; hypoconulid present but of variable proportions. C1/: short; triangular occlusal contour; one convex labial face, one concave lingual face; apex of the tooth directed posteriorly; surrounding cingulum, widening lingually; vertical anterior groove.
P2/–P3/: not represented in material collected; the presence of these two premolars is established from the type-species.
P4/: triangular, flanked by the sinus; dominant paracone from which arise two divergent crests directed towards the parastyle, one anterior and one lingual; occasional presence of small lingual relief, as rough outline of a protocone.
M1–2/: presence of para- and metalophs; sinuous labial edge; no continuity between the postparacrista and premetacrista; mesostyle of variable shape, ranging from two small, juxtaposed jugal swellings, to a straight mesostyle extending onto the side of the paracone; pronounced parastyle, metastyle absent (Fig. 9). M2/ wider than M1/, with metastyle better developed than parastyle, and a taller mesostyle.
M3/ not very reduced, with three ectoloph branches and metacone present; small cingulum on either side of protocone; sinus between metacone and postprotocrista.
Stehlinia gracilis mutans nov. ssp. (Plate 5a–j)
Stehlinia gracilis mutans nov. ssp. from Baraval, Coânac 1, Cavalé and Escamps: a BAR_SgmC.3.12, right C/1, labial view (left) and lingual view (right). b BAR_SgmA.2.10, fragment of left hemimandible with alveoli for I/3 et C/1 and with P/2–3. c BAR_SgmA.2.12, right P/4. d COA1_SgmA.1.2, left M/1. e CAV_SgmA.1.4, fragment of left hemimandible with M/2–3. f BAR_SgmC.3.4, right C1/, labial view (left) and lingual view (right). g ESC_SgmA.2.3, right P4/. h ESC_SgmA.2.4, holotype, right M1/. i ESC_SgmA.1.2, left M2/. j ESC_SgmA.1.3, left M3/. Stehlinia minor (Revilliod 1922) from Célarié standard and La Cantine 2: k CLS_SmA.2.1, fragment of left hemimandible with M/2–3. l CAN2_SmA.1.1, right M3/
Synonymy: 1973: Stehlinia sp. in de Bonis et al., tabl. 2a.
Diagnosis: subspecies barely larger than S. gracilis gracilis; P/3–4 more rectangular, emergence of a myotodonty in the lower molars; M3/ proportionally longer.
Derivatio nominis: from the Latin mutans: mutant; due to the emergence of myotodonty in the lower molars.
Holotype: ESC-ASgmA.2.4, right M1/ (Plate 5h), from the UM2 collections.
Type-locality: Escamps (MP 19), Lot, Phosphorites du Quercy, France.
Other localities: Guirolle rouge, Coânac 1, Célarié ocre, Célarié standard (MP 19), Pécarel, Tabarly (MP 20), Cloup d’Aural 1 (post MP 20), Coyrou 1-2 (MP 20/21), Ravet-Lupo (MP 21), La Plante 2, Mas de Got, Baraval, Cavalé (MP 22), Gardiol 3 (MP 23).
Material and measurements: see Appendix 4.
Description (only provided for the dental categories not observed for S. gracilis gracilis, and for those presenting different morphological character states):
I/1–3: presence only determined by the presence of alveoli of size approximate to that of P/2, meaning proportionally large in comparison to other teeth.
P/3–4: rectangular; ante- and postcingulids flanked by sinusoid, and curving cranially; dominant protoconid and metaconid occasionally apparent on the postparacristid of P/3, or even independent on P/4; flat posterior aspect with sharp edges; wide anterior and posterior edges.
M/1–2: nyctalodont to myotodont structure, with much reduced, cuspidate hypoconulid.
P2/: practically vestigial, judging from the alveolus.
P3/: triangular occlusal outline; convex anterior surface, flat posterior face; two relatively distinct roots, the lingual being the most reduced.
Variation within the subspecies S. gr. mutans: this species presents a slightly smaller form at La Plante 2 than at Mas de Got.
Comparison with the nominal subspecies: S. gr. mutans can be distinguished from S. gr. gracilis mostly by its morphology: squarer lower premolars (P/3–4); M/1–2 occasionally myotodont (1/1 at Ravet-Lupo, 1/4 at Cavalé, 2/30 at Baraval); more imposing entoconid in the wide talonid; M3/ proportionally longer (leaving the impression of larger size) (Fig. 10).
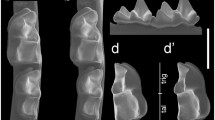
Line drawings of the morphological differences between two subspecies of Stehlinia gracilis: S. gracilis gracilis, a rectangular premolars, nyctalodont molars (left hemimandible bearing P/3–M/2, PRR SggA.2.5); b left M3/(MPR Sgg A.4.24). S. gracilis mutans nov. ssp., c right P/4 square (BARSgmA.1.11); d to the left, right M/1 nyctalodont (BAR SgmA.1.15), inverted; to the right, right M/1 myotodont (BAR SgmA.1.13, inverted); e left M3/(BAR SgmA.2.19)
Comparison of S. gracilis to other species of the genera: this species is clearly smaller than S. minor. Its P/3–4 are more rectangular, the lower molars sometimes myotodont, and the M3/ proportionally longer. S. gracilis can be distinguished from S. pusilla by the shorter lower molars and the more enclosed trigonids. The indeterminate species of this genus found at Le Garouillas, S. sp. indet. A, is clearly smaller.
Stehlinia minor (REVILLIOD, 1922) (Fig. 8b; Plate 5k, l)
Synonymy: 1922: Paleunycteris minor in Revilliod, p. 147–148, fig. 56
1979: Paleunycteris minor in Sigé et al. p. 49, 92
1981: Stehlinia minor (in parte) in Crochet et al., tabl. 2-2
1987: Stehlinia cf. minor in Remy et al., tabl. 1a-2a p. 177 and 180
Previous references (references where the citation of the species is taxonomically compatible with the present systematic propositions and the localities):
1973: Stehlinia minor in de Bonis et al., tabl. 2a.
1974: Stehlinia minor in Sigé, pp. 253–272
2006: Stehlinia minor in Sigé & Crochet, p. 196.
Amended diagnosis: species of intermediate size between the classic species S. gracilis and S. quercyi. Wider trigonid and ridge on the talon, less pronounced than on S. quercyi; upper molars less pinched than those of S. gracilis, and more transversely extended than what is typically seen in this genus.
Holotype (monotype): Q.P. 752, right hemimandible with the alveoli of C/1 and P/2–3 and bearing P/4–M/1–3, Revilliod (o.c.).
Type-locality: Old Quercy Collections (unknown locality, indeterminate age), France.
Reference population: Escamps (MP 19), Lot, Phosphorites du Quercy, France (Sigé 1974a, b).
Other localities: La Cantine 2, Clapassou (MP 17a), Perrière, Malpérié (MP 17b), Bouyssou 2 (MP 18), Rosières 3, Rosières 2, Coânac 1, Célarié standard (MP 19), Pécarel, Tabarly (MP 20). Material and measurements: see Appendix 4.
Description: the details provided in Sigé (1974) based on the material from Escamps will not be revised here, given the good representation of the species from this locality, and the morphological variability of its dentition.
Comparison: Importantly, the locality of Escamps is the only one in this study to have provided such a large proportion of specimens of this Stehlinia species as well as other species. It alone accounts for almost all the intraspecific variability seen in this species. Some rare differences were observed, notably on M3/ from La Cantine 2, which presents a less developed parastyle and a protocone with a wider base than in the Escamps sample, as well as the only specimen from Célarié standard, which has lower molars with wider basins and a smaller entoconid.
The size of S. minor (between those of S. gracilis and S. quercyi) is the feature that contributes most to the diagnosis for this species in comparison to other species of the genus. It is set apart by upper molars that are generally more transversely extended, with a talon that is less distinct, and an occlusal contour less pinched than in S. gracilis. The lower molars have a wider trigonid than S. quercyi.
Stehlinia quercyi (REVILLIOD, 1922) (Plate 6)
Stehlinia quercyi (Revilliod 1922) from Malpérié and Perrière: a MPR_SqA.1.1, right C/1, labial view (left) and lingual view (right). b PRR_SqA.1.2, fragment of right hemimandible with at least an alveolus for the incisor and the alveolus for C/1, and with P/2–3–4 and M/1. c PRR_SqA.1.7, right hemimandible with alveoli for I/1–2, C/1 and P/2 and with P/3–4 and M/1–2–3. d PRR_SqB.1.7, left C1/ labial view (left) and lingual view (right). e PRR_SqA.3.9, fragment of left maxillary with alveolus for P2/ and with P3–4/. f PRR_SqA.3.1, left M1/. g PRR_SqA.3.6, left M2/. h PRR_SqA.3.14, left M3/
Synonymy: 1922: Paleunycteris quercyi in Revilliod, p.145
1967: Paleunycteris aff. quercyi in Miguet, p. 111–113
1979: Paleunycteris quercyi in Sigé et al., p. 49, 92
Previous references: 1987: Stehlinia quercyi in Remy et al., tabl. 1a p. 177
Amended diagnosis: the largest species of the genus Stehlinia.
Holotype (monotype): Q.P. 727, right hemimandible presenting alveoli for /1–2 and bearing C/1–M/3, p. 145, Revilliod (o.c.), from the collections of the Naturhistorisches Museum Basel.
Type-locality: Old Quercy Collections (unknown locality, undeterminate age), France.
Reference population: Perrière (MP 17b), Tarn-et-Garonne, Phosphorites du Quercy, France, by assigning the type to this biochronologically well-situated population. Other localities: Clapassou (MP 17a), Malpérié (MP 17b).
Material and measurements: see Appendix 4.
Description: the mandible has a subvertical ascending ramus; a deep masseteric fossa on the oblique axis rising posteriorly; a strongly elevated ventral margin in relation with the horizontal ramus; a long angular apophysis facing posteriorly and capped with a ridge at one end; a better developed condyle medially; an angular apophysis deflected laterally (Fig. 8c). Two lower incisors: one antero lingual, one posterolabial.
C/1 barely taller than P/3; oblique cingulid strongly elevated anteriorly, widening lingually.
P/2 with pronounced surrounding cingulid, uniradiculate, of proportionally significant size (roughly half of P/3).
P/3 rectangular, longer and taller than P/4; continuous thick cingulid; flat posterior face, delimited by two sharp crests.
P/4 subsquare but morphologically similar to P/3, continuing homodont tendency from the anterior tooth row.
M/1–2: large nyctalodont molars; highly developed entoconid, of same volume as the hypoconid; cuspidate hypoconulid, reduced; M/1 occasionally submyotodont to myotodont (one example at Perrière); continuous, thick cingulids; M/2 trigonid larger than that of M/1 (Fig. 11).
M/3 typical of this genus, with a hypoconulid.
C1/: large; short lamella; wide surrounding cingulum, irregular on labial side; three aspects: one convex anterior, one labial, one lingual and more concave.
P2/ uniradiculate, of relatively significant size judging from alveolus.
P3/ with paracone and slight heel.
P4/ larger than P3/; marked postparacrista; pronounced space anterior to paracone; small protocone at the anterolingual corner.
M1–2/ typical of genus Stehlinia, with a sinuous labial edge; prominent mesostyle; thick and distinct pre- and postcingulum, situated respectively on the anterior and posterior sides of the protocone, the posterior as part of a nascent talon; postprotocrista attenuating before joining the base of the metacone; weak paraloph, but distinct metaloph.
M3/ also typical of the genus, with three ectoloph branches; sinus between metacone and postprotocrista; small cingula on anterior and posterior sides of protocone.
Comparison: it is much larger than the other species of this genus, such that its size constitutes a sufficiently distinctive feature. Of further note is the increased lingual opening and decreased width of the trigonids of the lower molars, as in S. rutimeyeri.
Remarks on the species Paleunycteris aff. quercy: The hemimandible fragment from the Old Quercy Collections, cited by Miguet (1967), presents a morphology typical of the genus Stehlinia. However, being notably smaller than S. quercyi and slightly larger than S. bonisi it is not possible to assign this specimen to one of the species reported in this work.
Stehlinia pusilla (REVILLIOD, 1922) (Fig. 12)
Synonymy: 1922: Paleunycteris pusilla in Revilliod, p. 149, fig. 58
1997: Stehlinia aff. pusilla in Sigé, p. 744
Amended diagnosis: species of Stehlinia characterized: by lower molars shorter and proportionally wider than common, as by: trigonid more closed than that of S. rutimeyeri; talonid less transversely developed; sinuous shape of the hemimandible.
Holotype (monotype): E.f. 993, left hemimandible bearing M/2 and alveoli of P/3 (1/2), P/4–M/1, M/3, p. 149, Revilliod 1922; Naturhistorisches Museum Basel.
Type-locality: Egerkingen (Switzerland), presumed age MP 14. Other localities: St-Maximin (MP 13), Phosphorites du Gard, France. Material and measurements: see Appendix 4.
Description: M/3 morphologically typical of the genus Stehlinia; slightly reduced talonid; strong entoconid with crest facing towards the basin and juxtaposed to a slightly cuspidate hypoconulid; pronounced cingulids.
Comparison: the type of S. pusilla which originally contained M/2–3 in a hemimandible now has only the M/2. Based on being equivalent in size b to that of the M/3 from St-Maximin, this would appear to correspond to the same species as in Egerkingen, given that both localities are close in age.
Remarks: the Cuzal specimens attributed to this species by Marandat et al. (1993) were shown to be larger after direct comparison with the type. Here they are assigned to the species S. alia nov. sp., described and compared further below.
Stehlinia rutimeyeri (REVILLIOD, 1922)
Synonymy: 1922: Paleunycteris rutimeyeri in Revilliod, p. 148 fig. 57.
Remarks on the diagnosis: the author describes and compares the only specimen at his disposal, without providing a detailed diagnosis. This work does not assign or provide any new specimens to this species.
Holotype (monotype): Ef. 992, left hemimandible bearing broken P/3, P/4–M/3, p. 147, Revilliod 1922, from the collections of the Naturhistorisches Museum Basel.
Type-locality: Egerkingen (Switzerland), presumed age MP 14.
Remark: despite a significant discrepancy in size, the morphological resemblance between the lower molars of this taxon and those of S. quercyi is noteworthy: with narrower trigonid; and teeth longer than those of other species in the genus.
Marandat et al. (1993) and Astruc et al. (2000) mention this species at the Cuzal locality (MP 13). Despite this, based upon comparisons drawn with the type, it seems that the teeth from this locality are larger. Thus, the Cuzal specimens are assigned here to the species S. revilliodi nov. sp. described later in this work.
Stehlinia bonisi SIGÉ, 1990 (Fig. 8d)
Synonymy: 1987: Stehlinia minor and cf. in Remy et al., tabl. 2a–3a p. 180 and 183
1998: Stehlinia minor in Sigé et al., p. 86
2006: Stehlinia minor in Sigé & Crochet, p. 196
Previous references: 1995: Stehlinia bonisi in Sigé, p. 106–109, text-fig. 1–7
2006: Stehlinia bonisi in Sigé & Crochet, p. 191–192
Original diagnosis: Stehlinia of average size; anterior orbital edge vertical and prominent; C1/ with reduced mesial groove; P2/ much reduced; P4/ with transversely extended, slightly pinched contour; reduced lophs on M1/ and more particularly M2/; distolingual corners of M2/ prominently displayed; C1/ with pronounced lingual–mesial ridge; lower labial teeth with unthickened, uncrenelated labial cingulum.
Holotype: (UM2) GAI 47, left hemimandible fragment partially preserving the ascending ramus, the alveoli of P/3 and bearing P/4–M/3, fig. 7, Pl. 1, Sigé (1990), from the collections of UM2.
Type-locality: Le Garouillas (MP 25), Lot, Phosphorites du Quercy, France.
Other localities: Aubrelong 1 (MP 21), La Plante 2, Baraval, Cavalé (MP 22), Belgarric, Ppcofi, L’Escoufle, (MP 25).
Material and measurements: see Appendix 4.
Description: Sigé (1995) has described this taxon.
Comparison: The species that is closest both in terms of morphology and dimensions to S. bonisi is S. minor. The species S. bonisi is slightly larger than specimens of S. minor. The alveoli of I/1–2 are not separated, and those of P/2–3 are smaller. On the C/1, the vertical groove is shallower. In a myotodont schema, the M/3 generally has a crestiform hypoconulid that is greatly reduced or more cuspidate, and set at the rear of the talonid basin (2/6 at Baraval). The P4/, more transversely extended, has a cingulum that is wider and more undulating, with notable median pinching to the posterior edge, and a small independent protocone. M1–2/ generally have a less clearly defined metaloph.
Stehlinia revilliodi nov. sp. (Plate 7a–i)
Stehlinia revilliodi nov. sp. from Cuzal and Chamblon: a CUZ-390, fragment of left hemimandible with P/2–3. b CUZ-393, right P/4. c CHA_SrA.2.11, left M/1. d CHA_SrA.3.5, left M/2. e CUZ-407, left C1/, labial view (left) and lingual view (right). f CHA_SrA.2.2, left P3/. g CHA_SrA.2.4, right P4/. h CHA_SrA.1.7, left M1/. i CUZ-415, holotype, left M2–3/. Stehlinia alia nov. sp. from Cuzal and St-Maximin: j CUZ-386, fragment of left hemimandible with alveoli for P/3–4 and with C/1 and P/2
Synonymy: 1983: Stehlinia sp. in Sigé & Legendre, tabl. 3 p. 212
1993: S. rutimeyeri in Marandat et al., p. 620, Pl. 1 fig. 7
2000: S. rutimeyeri in Astruc et al., table 1 p. 278
Diagnosis: intermediate size between S. minor and S. quercyi; lower molars shorter and with wider trigonid than S. minor; M2/ surrounded by a continuous postcingulum and with a closed protofossa at the base of the metacone.
Derivatio nominis: in honour of Pierre Revilliod, author of many significant works on fossil bats, notably from the Quercy.
Holotype: CUZ 415, left M2–3/ (Plate 7i), from the collections of UM2.
Type-locality: Cuzal (MP 13), Lot, Phosphorites du Quercy, France.
Other locality: Chamblon (MP 13), Switzerland. Material and measurements: see Appendix 4. N.B.: the material from Chamblon studied here comes from small samples collected by Dr. Bernard Sigé and M. D. Rigassi (Swiss geologist).
Description: P/2 biradiculate, positioned obliquely in the mandible; oval occlusal contour; thick surrounding cingulid; distinct pre- and postprotocristid.
P/3 biradiculate, smaller than P/4; thick surrounding cingulid, rising slightly anteriorly.
P/4 with wider cingulid posteriorly, ending in small swelling at the anterolingual corner.
M/1–2 with a greatly reduced hypoconulid, sometimes cuspidate but generally crestiform, jutting slightly posteriorly; entoconid distinct and tall, composed of three faces delimited by anterior, posterior and labial crests; M/2 with slightly more open trigonid and wider than M/1.
C1/ short; surrounding cingulum, slightly less prominent at the point of maximum curvature labially; very flat lingual face.
P3/ with a somewhat oval occlusal contour; single cusp situated at the anterolingual corner of the tooth; sharp posterior crest originating from the cusp; slight lingual basin; surrounded by pronounced cingulum.
P4/ triangular, composed of a tall paracone, a slight parastyle, a postparacrista, and a sinus flanking a pronounced lingual cingulum, occasionally developing an independent protocone; labial edge more or less curved.
M1–2/ large, with pronounced parastyle; M2/ with slightly developed talon bearing a thick cingulum; deep protofossa, closed by a postprotocrista connected to the base of the metacone; smaller postcingulum.
M3/ with ectoloph composed of three crests; lingual cingulum present; present postprotocrista until parastyle.
Comparison: the Chamblon specimens, in a very good state of preservation, are slightly larger than those from Cuzal. S. revilliodi nov. sp. is larger than S. rutimeyeri and S. minor. The lower molars have a trigonid that is less transversely compressed, and seem to be shorter than those of S. minor. The species S. revilliodi nov. sp. is larger than S. alia nov. sp., but P/2 is proportionally smaller and has an uninterrupted lingual cingulid; P/4 is less molariform, whereas M2/ has an uninterrupted postcingulum and a closed protofossa at the base of the metacone.
Stehlinia alia nov. sp. (Fig. 13; Plate 7j; Plate 8a–f)
Stehlinia alia nov. sp. from Cuzal and St-Maximin: a CUZ-388, fragment of left hemimandible with alveoli for C/1 and P/2, and with P/3–4. b CUZ-392, left hemimandible with alveoli for I/1–2–3, C/1, P/2–3, and with P/4 and M/1–2–3. c SMX_Sa1, right C1/, labial view (left) and lingual view (right). d SMX-PF02F, right M1/. e CUZ-385, holotype, left M2/. f SMX-PD01, right M3/. Stehlinia sp. B from Aubrelong 2: g ABL2_SspBA.1.1, right M1/
Synonymy: 1993: S. cf. pusilla in Marandat et al., p. 620, Pl. 1, fig. 8
2000: S. cf. pusilla in Astruc et al., table 1, p. 278
Diagnosis: species intermediate in size between S. pusilla and S. minor, and distinct from S. rutimeyeri due to the wider lower molars, the trigonids being more closed, and a crista obliqua connecting to the trigonid more lingually.
Derivatio nominis: from the Latin alius: different, to underline that this species is distinct from S. pusilla, to which it was initially thought to be close.
Holotype: CUZ 385, left M2/ (Plate 8e), from the collections of UM2.
Type-locality: Cuzal (MP 13), Lot, Phosphorites du Quercy, France.
Other localities: Aumelas, St-Maximin (MP 13).
Material and measurements: see Appendix 4.
Description: St-Maximin: M1/ with slight notch on the labial edge on either side of the mesostyle; pronounced parastyle; metaloph and paraloph both present; postprotocrista thinning out from the protocone and joining the postcingulum; heel with thick cingulum, extending to the anterior side of the protocone; wide space between preprotocrista and the side of the paracone.
M3/ typical of the genus with a cingulum on each side of the protocone, the posterior one being less well defined.
Cuzal: wide mental foramen, situated beneath C/1–P/2.
Three incisors very close together, as indicated by their alveoli.
C/1: not especially tall, with small alveolus; thick and very wide anterior cingulid with small swelling; absence of delimited sides.
P/2 uniradiculate and unicuspidate, with a cingulid more or less anterolingually and posterolabially developed.
P/3 slightly smaller than P/4; biradiculate; rectangular in occlusal outline; central protoconid, with two sinuous crests connecting the antero- and posterolingual corners, and forming a slight anterior relief; cingulid thick and wide, surrounding the tooth.
P/4 biradiculate but wider posteriorly; anterior relief (paraconid) potentially elevated; existence of a posterior surface to the protoconid.
P/2–P/4: three surfaces, one oblique and lingual, one convex and labial, one flat and posterior strongly marked by wear.
M/1–2: large teeth with nyctalodont structure; narrow trigonid; extended talonid with very pronounced entoconid, extending to the middle of the basin and bearing a marked labial crest that delimits the anterior from the posterior aspects; hypoconulid jutting posteriorly, practically crestiform; wide cingulids; M/1 with narrower trigonid than M/2, slightly more open; small lingual ridge between the base of the paraconid and the metaconid; anterolingual corner of the talonid basin very deep (mastication effect).
M/3 small compared to M/1–2, but with proportionally less reduced talonid: smaller entoconid and hypoconulid only; trigonid slightly less closed than on M/2.
C1/: flat lingual and labial faces; convex anterior surface, divided by a groove; surrounded by a cingulum, more pronounced lingually.
Upper molars: only one M2/ found at Cuzal, no heel labial notch in front of the cuspidate mesostyle; parastyle present but not metastyle; paraloph meeting the lingual edge of the protofossa; shorter metaloph meeting the posterior margin; cingulum present across the base of the protocone; pre- and postprotocrista respectively connected to the pre- and postcingulum. Aumelas: M1/ of compatible size to M2/ from Cuzal; postprotocrista terminated at level of metaloph; postcingulum wide and slightly cuspidate lingually, forming an incipient heel (Fig. 13).
Comparison: the M2/ from Cuzal seems small in comparison to the M1/ from St-Maximin, and in comparison to the lower molars present at Cuzal and to the proportions observed for the other species of the genus. It seems reasonable to suggest that this specimen represents the smallest known form connected to this species. Other Cuzal specimens are slightly larger than St-Maximin ones. The lower molars from Cuzal are wider, with trigonids more closed, and cristae obliqua more oblique (so meeting the trigonid more lingually) than in S. rutimeyeri.
Stehlinia alia is clearly smaller than S. minor from Perrière and displays morphological differences such as: P/3–4 with a more oval occlusal contour bearing an anterior relief, as weaker cingulids; M1/ with a less-defined labial edge, a postprotocrista that is only at the level of metaloph rather than the postcingulum. Finally, it differs from S. pusilla its larger M/2 and deeper horizontal ramus; it differs from S. sp. A from St-Maximin, and from S. revilliodi nov. sp. from Cuzal, by its smaller size.
Stehlinia sp. A
Previous references: 1974: Stehlinia (sp.) in Sigé: p. 270.
1997: Stehlinia sp. A in Sigé: p. 741–743, fig. 7–8.
Locality: St-Maximin (MP 13), Phosphorites du Gard, France.
Material and measurements: see Appendix 4.
Remarks: with no new specimens being assigned to this taxon, the description and comparisons by Sigé (1997) still stand. As a result, these remain complete.
Stehlinia sp. B (Plate 8g)
Synonymy: 1987: Stehlinia sp. in Remy et al., tabl. 1a p. 177
Locality: Aubrelong 2 (MP 17a), Lot, Phosphorites du Quercy, France.
Material and measurements: see Appendix 4.
Description: one single large and massive M1/, with wide and thick cingulum, bisected on the flank of the protocone; open protofossa with postprotocrista decreasing posteriorly, forming a small projection.
Comparison: This species is similar to S. revilliodi nov. sp. from Cuzal in that it is larger than other Stehlinia species of about the same age, except for S. quercyi, and it can be distinguished by its larger size, squarer occlusal molar contour, and more pronounced cingula.
Remarks on the systematic attribution of the genus Stehlinia: This work contributes to a complete review of the different morphological types of Chiroptera that have been described since the middle Eocene, and thus helps to better understand the affinities between the main groups. The genus Stehlinia, relatively undocumented in the Quercy faunas compared to the genera Palaeophyllophora, Vespertiliavus or Hipposideros (Pseudorhinolophus), has led to several familial reconsiderations. Its attribution to the Palaeochiropterygidae within the Vespertilionoidea as formalized by Sigé (1997) captures the strong similarities in dental morphology shared not only with the older genera (Palaeochiropteryx, Cecilionycteris and Matthesia), but also with more modern Vespertilionidae. These are, notably, the less restricted dental formula, the more pronounced entoconid, the sinuous labial edge, the absence of heels on the upper molars and the less reduced M3/3. In general, this group has derived features, appearing rather late in the fossil record (the first occurrence corresponds to the reference level MP 19 with the deposit at Batut; Muratet, 1985). The proposed classification by Sigé (1997) seems to be confirmed by the new data, and thus is used here.
Superfamily Emballonuroidea DOBSON, 1875 (WEBER, 1928)
Family Emballonuridae GERVAIS (in de Castelnau, 1855)
Gervais (1855) considers the genus Emballonura to be representative of a new lineage, distinct from those already recorded in extant Chiroptera, and thus creates the tribe Emballonurina. Thus, through systematic ascension, the family Emballonuridae appears ever providing explicit characteristics. Other more recent authors have qualified this family based upon the observation of extant material, notably Miller (1907) who mostly evokes the cranial and postcranial diagnostic criteria. “The members of the family are recognizable by their normal teeth, free premaxillaries, well-developed postorbital processes, the reduced condition of the index finger, and the primitive structure of the shoulder joint. Externally they may be distinguished by the combination of slender leg with reflexed proximal phalanx of third finger. In all the known genera the tail perforates the interfemoral membrane and appears on its upper side distinctly back from the edge”. Current geographic distribution: tropical regions of both hemispheres and Samoa (east Pacific Ocean).
Genus Vespertiliavus SCHLOSSER, 1887
Dental formula: I 1?/3, C1/1, P3/3, M3/3 Amended diagnosis (the author having failed to provide a precise diagnosis for this genus, only the dental characteristics indicated by Revilliod (date), based upon more extensive, albeit poorly dated, material are taken into account here): the middle lower incisor is the smallest; P/2 uniradiculate, with a triangular crown as long as that of P/4; P/3 remains biradiculate but with smaller crown, set more or less transversely on the alveolar edge; independent conical tip of molar trigonid cusps; talonid wider and as long as the trigonid; horizontal ramus height below M/3 equal to that of P/4, reaching maximum height under P/4 before rapid decrease anteriorly; high coronoid apophysis, with somewhat straight anterior crest.
Type-species: Vespertiliavus bourguignati (Filhol 1876), p. 45–48, Pl. 7 fig. 5–8.
Other species described: V. wingei; V. schlosseri; V. gracilis; V. lapradensis; V. gerscheli; V. disjunctus disjunctus nov. sp., nov. ssp.; V. disjunctus nauzensis nov. sp., nov. ssp.; V. (Sigeia) lizierensis nov. sgen., nov. sp.; V. (Sigeia) recens nov. sgen., nov. sp.
Distribution: from middle Eocene (MP 13) to upper basal Oligocene (MP 25) in western Europe (France).
Vespertiliavus bourguignati (FILHOL, 1876) (Plate 9)
Vespertiliavus bourguignati (Filhol 1876) from La Bouffie: a BFI_VbA.3.14, right P/2. b BFI_VbA.3.19, left P/4. c BFI_VbA.4.13, left M/1. d BFI_VbA.4.12, left M/2. e BFI_VbA.4.16, left M/3. f BFI_VbA.3.4, left P4/. g BFI_VbA.1.5, left M1/, morphotype 1. h BFI_VbA.1.8, right M1/, morphotype 2. i BFI_VbA.2.5, right M2/, morphotype 1. j BFI_VbA.2.10, right M2/, morphotype 2. k BFI_VbA.1.18, right M3/
Synonymy: 1876: Vespertilio bourguignati in Filhol, p. 45, Pl. 7 fig. 5–8
1885: Vespertilio bourguignati in Lydekker, p. 13
1887: Vespertiliavus brongniarti in Schlosser, p. 72
1891: Vespertiliavus bourguignati in Zittel, p. 577
1896: Vespertiliavus bourguignati in Roger, p. 28
1898: Vespertiliavus bourguignati in Trouessart, p. 124
1904: Vespertiliavus bourguignati in Trouessart, p. 89
1919: Vespertiliavus bourguignati in Revilliod, p. 81, 105, fig. 23, 28, Pl. 2, fig. 13
1979: Vespertiliavus bourguignati in Sigé et al., p. 46, 92
1981: Vespertiliavus wingei in Crochet et al., tabl. 2-2
1987: Vespertiliavus cf. schlosseri in Remy et al., tabl. 1a, p. 177
Original diagnosis: posterior lower premolar shorter than M/3, cingulum less pronounced at the posterolabial corner, lingual cingulum absent and lingual aspect of the cusp slightly flattened. M3/ shorter than in V. wingei.
Amended diagnosis: of intermediate size between V. wingei and V. schlosseri. Nyctalodont lower molars and upper molars with rectangular occlusal contour and displaying talon of highly variable form (from straight to sinuous).
Syntypes: Filhol does not provide a type-specimen. He features a maxillary and a hemimandible based on very schematic and unrepresentative drawings of these specimens with no inventory number (Plate 7; Figs. 5, 6, 7, 8), from the collections of the Museum d’Histoire Naturelle de Genève.
Type-locality: multiple localities (Caylux, St-Antonin, Lamandine-Haute), Old Quercy Collections (indeterminate age), France.
Reference population: La Bouffie (MP 17a), Lot, Phosphorites du Quercy, France Material and measurements: see Appendix 4.
Description: P/2 longer than P/4 but narrower, suboval occlusal contour with the anterior part of the tooth lingually orientated; two crests beginning at the paracone joining the anterior and posterior extremities of the tooth; apex directed lingually.
P/3: not observed.
P/4: occlusal contour more rectangular than P/2 but relatively similar morphology, small swelling in the anterior region connected to the postprotocristid; lingual and labial edges generally sinuous.
M/1–2: nyctalodont molars; trigonid closed and wide; M/1 trigonid with distance between paraconid and protoconid longer than between metaconid and protoconid; pronounced entoconid, crestiform hypoconulid, extending posteriorly.
M/3 without hypoconulid, very reduced talonid.
P4/ triangular, long labial edge, parastyle and protocone somewhat developed and separated from the paracone by a sinus, significant talon hooked posteriorly, very slight postcingulum.
M1–2/ with a pronounced parastyle and a cuspidate metastyle, particularly on M1/; contour and development of the heel very variable: linear posterior edge and short heel or oblique edge pinched as it gets further from the lingual edge or sinuous margin generally more developed lingually; cingulum always thick regardless of morphotype and generally connected to the postprotocrista; two forms on M2/: either there is no connection or there is a connection and an uninterrupted cingular ridge on the lingual side of the protocone; indentation of the labial edge at the level of the paracone more pronounced on M2/.
M3/ slightly reduced, three ectoloph branches with beginnings of a fourth; pronounced parastyle, postprotocrista separated from the metacone by a sinus.
Comparison: V. bourguignati is smaller than V. wingei with narrower, longer P/2. The upper molars of V. wingei are squarer and very large, with a less variable talon; the contour of the protocone of M3 from La Bouffie is squarer, further separated from the labial part of the tooth by a slight anteroposterior pinching. In comparison to V. schlosseri, V. bourguignati is larger and does not have nyctalodont lower molars.
Remarks on Vespertiliavus cf. bourguignati described by Barghoorn (1977): During the study of several specimens that he attributes to the genus Vespertiliavus, Barghoorn (1977) misinterpreted the dental nomenclature used by Révilliod (1920): he considered P1/1 as being the most anterior premolar, whereas Revilliod used this number to designate the most posterior premolar. Therefore, the comparisons drawn by Barghoorn between the dimensions of his dental rows (P/1–M/3) and those of Revilliod have no value given that they do not pertain to the same teeth. The designation of one of the specimens as V. cf. bourguignati, pending the creation of a new species, is no longer applicable as it stands and requires reexamination of the material.
Vespertiliavus wingei REVILLIOD, 1920 (Plate 10)
Vespertiliavus wingei Revilliod 1920 from Perrière: a PRR_VwC.3.18, left C/1 labial view (left) and lingual view (right). b PRR_VwC.2.4, left hemimandible with alveoli for I/1–2–3 and with C/1, P/2–3–4, M/1–2–3. c PRR_VwB.3.10, left P4/. d PRR_VwB.1.5, right M1/, morphotype 1–2. e PRR_VwB.2.8, right M2/, morphotype 2. f PRR_VwB.3.6, right M3/
Synonymy: 1971: Hipposideros (Pseudorhinolophus) sp. in Menu & Sigé, fig. 2
1979: Vespertiliavus wingei in Sigé et al., p. 46, 92
Previous references: 1981: lV. wingei in Crochet et al., tabl. 2-2
1987: Vespertiliavus wingei in Remy et al., tabl. 1a p. 177
Original diagnosis: Mandible: anterior premolar as tall as it is long, with a convex labial aspect, reaches the same length as the posterior premolar. Posterior premolar as long as M/3, cingulid very flared at the posterolabial corner. Maxillary: M3/ with anterior triangle as developed as in M2/.
Amended diagnosis: very large species of the genus Vespertiliavus; nyctalodont lower molars; long M/3; upper molars very large, with square occlusal contour, bearing a talon with a straight posterior edge; M3/ clearly longer than that of V. schlosseri.
Lectotype: Revilliod (1920) having featured a maxillary and a hemimandible, the choice was made here to designate as lectotype the specimen Q.P. 855, a relatively complete fragment of the left maxillary bearing P3–4/ and M1–2–3/ figured Pl. III, fig. 11, from the collections of the Naturhistorisches Museum Basel.
Type-locality: Old Quercy Collections (indeterminate age), France; the locality of Lamandine without further details (lower L.: upper Eocene, upper L.: Oligocene; Sigé et al. 1979, p. 89) is provided for the type-hemimandible.
Reference population: Perrière (MP 17b), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Célarié standard (MP 19).
Material and measurements: see Appendix 4.
Description: Mandible: subvertical ascending ramus rising quickly posterior to M/3, and slightly laterally inclined; masseteric fossa orientated along the axis of the base of the ascending ramus, thus slightly oblique; thickish condyle on the axis of the tooth row; angular apophysis, much extended posteriorly; horizontal ramus rising anteriorly (anterior to P/4; Fig. 14).
I/1–3: not found in the study material but three alveoli indicate their presence anterior to C/1. Narrow, transversely extended, very labially compressed, roughly equivalent in size.
C/1: tall, thin and directed lingually; no labial cingulid; lingual face with widened cingulid anteriorly and posteriorly.
P/2 unicuspidate, with oval occlusal contour, as long as P/4 but less tall; slight orientation of the cusp lingually; composed of a central apex from which two anterior and posterior crests connected to the lingual corners of the tooth arise; surrounded by a thick cingulid.
P/3 narrow, much smaller than the other premolars; two roots positioned obliquely on the tooth; thick cingulid, oblique in labial view (higher anteriorly) and rising posteriorly.
P/4 narrow, bicuspidate, surrounded by a thick cingulid, sinuous in labial view and slightly oblique as in P/3, rising towards the back of the tooth at the edge of a slight basin.
M/1–2: nyctalodont molars with wider talonids than trigonids; wider trigonid on M/2 than M/1; much more pronounced entoconid, crestiform hypoconulid projecting posteriorly; trigonid much taller than talonid.
M/3: narrower and without hypoconulid; a more enclosed trigonid than on M/2; much shallower talonid than on M/1–2.
P4/ composed of a dominant paracone and a jutting postparacrista meeting the posterior edge; some significant development of a posterior parastyle, protocone and heel; uninterrupted cingulum running from the parastyle to the metastyle, occasionally somewhat pinched at inflexion point of the posterior edge; paracone separated from the more anterior reliefs by a sinus.
M1/ narrower anteriorly than posteriorly; strong parastyle; oblique mesostyle, forming a projection on the crenullated labial edge; high prominent protocone with a preprotocrista connected to the parastyle, and a short postprotocrista ending abruptly in a projection, leaving the protofossa mostly open; short postcingulum, thick and oblique on the metacone’s posterior flank; talon with straight posterior edge, with a basin and well-developed thick ridge, extending up the posterior flank of the protocone; two morphotypes coexist depending on the presence and type of connection between this ridge and the postprotocrista: morphotype (1), connection between these two crests at the level of the projection followed by attenuation of the postprotocrista towards the base of the metacone; morphotype (2), no connection, longer postprotocrista curved at the extremity, ridge dwindling before reaching the top; M2/ with same morphology as M1/, with more indented labial edge at the level of the paracone (Fig. 15). M3/ slightly reduced with three ectoloph branches and the beginnings of a fourth; very prominent mesostyle on the labial edge; pronounced parastyle; protocone connected to the parastyle; postprotocrista separated from the metacone by a slight sinus.
Comparison: in comparison to the Lamandine specimens, the Perrière species appears to be slightly smaller and has proportionally longer P/2–3.
This species can be distinguished from others by its larger size and its massive, longer upper molars. Furthermore, it can also be distinguished from V. bourguignati by a straight rectilinear heel and M/3 with long talonid.
Vespertiliavus schlosseri REVILLIOD, 1920 (Plate 11)
Vespertiliavus schlosseri Revilliod 1920 from St-Lizier: a SLI_VsD.3.25, right C/1, labial view (left) and lingual view (right). b SLI_VsD.3.1, right P/2. c SLI_VsA.4.7, right P/4. d SLI_VsA.2.6, left M/1. e SLI_VsA.1.19, right M/2. f SLI_VsA.2.22, left M/3. g SLI_VsD.4.8, right C1/, labial view (left) and lingual view (right). h SLI_VsC.1.4, left P4/. i SLI_VsD.1.3, left M1/, morphotype 1. j SLI_VsC.3.8, left M1/, morphotype 2. k SLI_VsC.4.17, left M2/, morphotype 1. l SLI_VsC.4.14, left M2/, morphotype 2. m SLI_VsD.2.25, left M3/. n SLI_VsD.2.5, right M3/
Synonymy: 1973: Vespertiliavus indet. 1 in de Bonis et al., tabl. 2a
1979: Vespertiliavus schlosseri in Sigé et al., p. 46, 92
1981: Vespertiliavus cf. wingei in Crochet et al., tabl. 2-2
1987: Vespertiliavus cf. schlosseri et sp. in Remy et al., tabl. 1a p. 177
1988: Vespertiliavus cf. schlosseri in Sigé, p. 85-87, text-fig. 15–21
Previous references: 2006: Vespertiliavus schlosseri and V. sp. A in Maitre et al., p. 116 and 118, fig. 5a
Original diagnosis: posterior premolar same length as M/3, with cylindrical cusp, cingulum with flared posterolabial corner, internal cingulum worn in centre. Very wide molar talonids. Comparatively short M3/, somewhat transversely extended molar triangles. Labial anterior corner of P4/ cingulum open and rounded; pronounced median tip of lingual cingulum; posterior talon more rounded.
Amended diagnosis: species of intermediate size between V. bourguignati and V. disjunctus; lower molars with nyctalodont to submyotodont structure; rectangular upper molars, talon with straight posterior edge; short M3/.
Lectotype: amongst the various specimens featured by Revilliod (1920) for V. schlosseri, the specimen from the collections of the Muséum de Genève, Quercy 1: a fragment of the right hemimandible bearing P/4–M/1 (Revilliod 1920; fig. 29 p. 90), was chosen here as the lectotype for this species.
Type-locality: Old Quercy Collections (unknown locality, indeterminate age), France.
Reference population: St-Lizier (MP 16), Tarn, Phosphorites du Quercy, France.
Other localities: Le Bretou (MP 16), Lébratières 1, La Cantine 2, Les Pradigues (MP 17a), Perriqre, Malpprip, Coyrou 3, Pech d’Isabeau (MP 17b), Thpron, Monteils (MP 18), Escamps, Palembert (MP 19), Tabarly (MP 20).
Material and measurements: see Appendix 4.
Description: C/1 very thin; oblique lingual cingulid, more elevated anteriorly; apex slightly turned lingually, widening anteriorly and posteriorly; posteriorly cuspidate and flanked by a sinus.
P/2 with oval contour slightly extending anteriorly; surrounded by pronounced cingulid, generally thicker in two points posteriorly.
P/4 more rectangular; generally shorter than P/2.
M/1–2 nyctalodont to submyotodont; crestiform hypoconulid; pronounced entoconid; M/2 with transversely extended, less open talonid.
M/3 with crista obliqua lingually joining the trigonid and thus considerably reducing the talonid basin.
C1/ tall, with a pronounced internal cingulum with cusp and a more lingual anterior part; two surfaces: a flat lingual face and a convex labial aspect. P4/ with more or less developed parastyle and protocone, separated from paracone by a sinus; talon basin well developed and usually with a strong cingulum.
M1/ large; uncuspidate metastyle, extended posterolabial corner; variation of the connection between the postprotocrista and the cingulum of the heel: (1) short postprotocrista, connection present; (2) postprotocrista slightly longer ending in projection and diminished heel cingulum on protocone side; preponderance of morphotype 1 on M1/ and morphotype 2 on M2/ (Fig. 16). Presence of a vertical groove between the protocone and the heel, making the lingual edge bulbous; posterior edge of the talon mainly straight with lingual widening of the heel on some specimens; indentation of the M2/ labial edge more pronounced than on M1/; thick postcingulum starting at the base of the metastyle and running directly below the metacone.
M3/ with three ectoloph branches, sometimes displaying beginnings of a fourth; sinus present between postprotocrista and metacone.
Comparison: at Monteils the only specimen attributed to V. schlosseri is a left M/1 with a morphology typical of the genus Vespertiliavus, but showing a submyotodont structure. This is also the case at Pech d’Isabeau with 2 of 4 teeth, at Escamps with 1 of 2 teeth and at St-Lizier with 10 teeth of 85.
The P4/ has a heel that is less well cut than those of V. bourguignati. The upper molars are significantly less massive or square than those of V. wingei and V. bourguignati, and have a heel posteriorly rectilinear; in contrast to the two big species of this genus: it has an uninterrupted cingulum on the lingual edge of the heel, a marked vertical groove between the heel and the protocone, and its M3/ is shorter. In contrast to the smaller species, it is distinguished by its submyotodont molar structure.
Vespertiliavus gracilis REVILLIOD, 1920 (Plate 12a–l)
Vespertiliavus gracilis Revilliod 1920 from Perrière: a PRR_VgC.3.7, left C/1, labial view (left) and lingual view (right). b PRR_VgD.3.11, fragment of left hemimandible with alveoli for I/1–2–3, and with broken C/1 and P/2. c PRR_VgD.3.9, fragment of left hemimandible with alveoli for C/1 and P/2, and with P/3–4. d PRR_VgD.2.7, fragment of left hemimandible with alveoli for C/1, P/2–3, and with P/4 and M/1–2–3. e PRR_VgC.3.11, left C1/, labial view (left) and lingual view (right). f PRR_VgA.4.16, left P4/. g PRR_VgA.1.1, left M1/. h PRR_VgA.1.11, left M1/. i PRR_VgA.1.14, left M1/. j PRR_VgA.2.2, left M2/. k PRR_VgA.2.5, left M2/. l PRR_VgC.2.5, left M3/. Vespertiliavus disjunctus nauzensis nov. ssp. From Lébratières 15, La Pize (1,2), Tabarly and La Nauze 1: m LEB15_VdnA.2.7, right C/1, labial view (left) and lingual view (right). n LEB15_VdnA.2.5, right P/2. o LEB15_VdnA.2.4, left P/4. p LEB15_VdnA.2.1, left M/1. q PIZ_VdnA.1.3, right M/2. r LEB15_VdnA.2.3, left M/3. s LEB15_VdnA.1.3, right C1/, labial view (left) and lingual view (right). t TAB_VdnA.1.1, holotype, left M1/. u NAU1_VdnA.1.1, right M2/. v LEB15_VdnA.1.2, right M3/
Synonymy: 1973: Vespertiliavus cf. gracilis in de Bonis et al., tabl. 2a
1979: Vespertiliavus gracilis in Sigé et al., p. 46, 92
1981: Vespertiliavus cf. gracilis (pro parte) in Crochet et al., tabl. 2-2
1987: Vespertiliavus gracilis et cf. in Remy et al., tabl. 1a-2a p. 177 et 180
1998: Vespertiliavus cf. gracilis in Sigé et al., p. 87
Previous references: 2006: Vespertiliavus gracilis in Maitre et al., p. 117, fig. 5a
2006: Vespertiliavus gracilis in Sigé & Crochet, p. 194
Original diagnosis: posterior premolar shorter than on M/3, anterior premolar as long as anterior molars, relatively squat and more laterally compressed than in V. wingei. Posterior talon of P4/ slightly more transversely developed than in V. schlosseri, median cuspid/swelling of the lingual cingulum is rudimentary. M3/ very short, posterior crest of the mesostyle almost as long as the crest connecting the mesostyle to the paracone.
Amended diagnosis: intermediate size between V. (Sigeia) lizierensis and V. disjunctus; trigonid relatively open for Vespertiliavus; on M3/ much extended preparacrista; pronounced posterior cusp/cuspid on C1/.
Lectotype: specimen designated as species lectotype is that featured by Revilliod: Q.P. 977 (p. 87, fig. 26, from the collections of the Naturhistorisches Museum Basel), a relatively complete fragment of the left hemimandible showing the alveoli of C/1, P/3, M/3 and bearing P/2, P/4–M/1–2.
Type-locality: Old Quercy Collections (unknown locality, indeterminate age), France.
Reference population: the population of the Bretou was considered by Sigé (1988) as the reference population for V. gracilis. However, with the material gathered in this study, it seems that the species at Bretou and in other localities is a larger one: V. disjunctus disjunctus nov. sp., nov. ssp. The reference population attributed here to V. gracilis is the one from Perrière (MP 17b), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Lavergne (MP 16), La Bouffie, Aubrelong 2, Trifon (MP 17a), Malpérié, Pépénut, Sorcières (MP 17b), Théron, Gousnat (MP 18), Guirolle rouge, Rosières 2, Lostange, Guirolle blanc (MP 19), Tabarly (MP 20), Aubrelong 1, Ravet déblais, Ravet-Lupo, Escabasse 2 (MP 21), La Plante 2, Mas de Got, Baraval, Cavalé, Lébratières 12–13 (MP 22), Gardiol 3 (MP 23).
Material and measurements: see Appendix 4.
Description: I/1–3: presence indicated by three alveoli very compressed anterior to C/1.
C/1: typical of Vespertiliavus, with a pronounced cingulid on the lingual side of the tooth.
P/2 uniradiculate, with an oval occlusal contour surrounded by a thick cingulid; apex slightly directed lingually, from where originate two crests: one anterior, one posterior.
P/3 biradiculate, with roots variably oblique in tooth-row; surrounding cingulid, rising slightly posteriorly.
P/4 with pointed anterior edge, and wide, straight posterior edge; surrounding sinuous cingulid; dominant protoconid with two sinuous crests meeting the lingual extremities of the tooth; pronounced basin posterior to the protoconid, reaching to the labial side.
M/1–2 mainly nyctalodont and also submyotodont or even myotodont; wide talonid; pronounced entoconid (Fig. 17a–c).
Line drawings of the variability of dental morphology within the species Vespertiliavus gracilis: different structural types for the lower molars: a nyctalodont (right M/2, CAV_VgA.1.16); b submyotodont (right M/1, CAV_VgA.1.14); c myotodont (M/2, CAV_VgA.1.23); d connection between trigone and heel for the upper molars: d postprotocrest/talon (right M2/, PRR_VgB.2.6); e no connection, long postprotocrest (right M2/, PRR_VgB.2.1). Three morphotypes for the heel on the upper molars: f posterolingually extended heel (left M1/, inverted, RR_VgA.1.11); g linear posterior edge (left M1/, inverted, PRR_VgA.1.4), h lingually wider posterior edge (left M1/, inverted, PRR_VgA.1.12)
M/3 with very small talonid and no hypoconulid.
C1/ very thin; convex labial aspect with shallow cingulum more pronounced at front and rear of tooth, developing a pointed anterior swelling and a thick, wide posterior cingulum on the flat lingual face, unconnected to one another.
P4/ typical of the genus; long labial edge; dominant paracone; prominent postparacrista; somewhat developed parastyle and protocone, separated from paracone by a sinus; quite large heel composed of a basin delimited by a cingulum; the posterior margin of the tooth more or less pinched at the junction between heel and postcingulum.
M1–2/ with sinuous labial margin, the indentation at the paracone level being more pronounced on M2/; well-developed parastyle; prominent metastyle; squat protocone, short postprotocrista leaving the protofossa well open. On M1/, thick cingulum on heel always connected to postprotocrista; on M2/ two morphotypes: (1) identical to M1/, (2) longer postprotocrista and reduced cingulum extending to the flank of the protocone (Fig. 17d, e). Several shapes of heel on M1/: with straight posterior edge (such as M2/), or widened lingually, sometimes ending in a point greatly extended posterolingually (Fig. 17f–h).
M3/ transversely extended, with three ectoloph branches, of which the premetacrista is almost as long as the postparacrista; pronounced parastyle; independent mesostyle, clearly offset posteriorly.
Comparison: at Cavalé, 5 teeth of 22 are myotodont and 2 submyotodont whereas the remaining are nyctalodont. M3/3 are shorter, particularly M3/. The edges of the protofossa are less flared in specimens from Perrière than Bouyssou 1, Ravet-Lupo, Aubrelong 1 and Cavalé. In these same localities, P4/ has a larger heel and its posterior edge is not pinched. These observations are evidence of local intraspecific variations.
The specimens from La Bouffie are slightly larger than those from Perrière. This could be explained by the difficulty in determining the specimens belonging to the different species of Vespertiliavus found at La Bouffie. The overlapping gaps between their sizes make taxonomic determination much more difficult for the extreme forms.
Clearly, conforming to the rest of the fauna, the size of V. gracilis from Ravet is larger than that of the population from Aubrelong 1, and that of Mas de Got is larger than at La Plante 2.
In comparison to V. disjunctus nov. sp., this species has a proportionally longer P/4, M/1 with the trigonid extending anteriorly, and M/2–3 with narrower trigonid, particularly in the case of M/3; the talonid is proportionally smaller except on M/3, where V. disjunctus nov. sp. has the smaller talonid. The M3/ of V. gracilis has a larger preparacrista, giving an extended appearance to the tooth. The posterior cusp/cuspid on C/1 is more pronounced in V. gracilis and not as distinct in V. disjunctus nov. sp., with C/1 being markedly thinner and straighter.
The size of V. gracilis is clearly greater than that of V. (Sigeia) lizierensis nov. sgen., nov. sp.; P/2 and P/4 are both the same size and P/3 is prominent, whereas V. (Sigeia) lizierensis nov. sgen., nov. sp. has a much less prominent P/2 than P/4 and no P/3; its lower molars have a significantly more open trigonid, which is more transversely reduced. The upper molars of V. (Sigeia) lizierensis nov. sgen., nov. sp. are different in many respects (see this species).
Vespertiliavus lapradensis (SIGÉ, 1990)
Synonymy: 1990: Vespertiliavus lapradei in Sigé, p. 11–16
Previous references: 1993: Vespertiliavus lapradensis in Marandat, p. 618, Pl. 1, fig. 6
2000: Vespertiliavus lapradensis in Astruc et al., table 1, p. 278
2006: Vespertiliavus lapradensis in Sigé & Crochet, p. 195
Original diagnosis: Vespertiliavus smaller than the smallest named species (V. gracilis).
Holotype: Fragment of right hemimandible, LAP 158, showing the alveoli of the incisors and P/2 and bearing C/1 and P/3–M/3, well preserved, moderately worn, fig. 5, p. 12 (Sigé, 1990), from the collections of UM2.
Type-locality: Laprade (MP 14), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other locality: Cuzal (MP 13), Lot, Phosphorites du Quercy, France.
Material and measurements: see Appendix 4.
Remarks: the descriptions are those given by Sigé (1990) and Marandat et al. (1993); no other specimens could be assigned to this species in this study.
Vespertiliavus gerscheli SIGÉ, 1990
Synonymy: 1987: Vespertiliavus sp. Remy et al., tabl. 4a p. 186
Previous references: 1995: Vespertiliavus gerscheli in Sigé, p. 80–88, text-fig. 2–8.
2006: Vespertiliavus gerscheli in Sigé & Crochet, p. 194
Original diagnosis: somewhat large Vespertiliavus, larger than V. gracilis and V. lapradensis, smaller than other classic species. Strong variability in the lower molars, nyctalodont or myotodont, and the upper molars, with or without a rudimentary hypocone.
Holotype: (UM2 collection) GAR 2403, fragment of right maxillary bearing worn P4/–M2/, fig. 1, Pl. 1 (Sigé, 1990).
Type-locality: Le Garouillas, indurate facies and clay facies (MP 25), Lot, Phosphorites du Quercy, France.
Measurements: see Sigé (1995, p. 81).
Remarks: Sigé (1990, 1995) gives the characteristics of this species and compares it to the other known species of the Vespertiliavus genus. None of the fossil remains examined in this study indicates the presence of this species in the other localities studied.
Vespertiliavus disjunctus nov. sp.
Remarks: within this species, two subspecies must be recognized, distinguished essentially by their size. The specimens from Le Bretou (MP 16) are smaller than those observed from reference level MP 20 onwards (Tabarly, Cloup d’Aural 1, la Nauze 1 and Lpbratiqres 15).
They constitute the nominative new subspecies V. disjunctus disjunctus, which encompasses the material from reference level MP 16 to reference level MP 19, whereas the new subspecies V. disjunctus nauzensis groups those of reference level MP 20 to MP 22.
Diagnosis: same as subspecies V. disjunctus disjunctus (see below).
Derivatio nominis: from the Latin disjunctus: distinct, due to the erroneous/unavailable previous association of part of this material with the smaller species V. gracilis.
Vespertiliavus disjunctus disjunctus nov. sp, nov. ssp. (Fig. 14b)
Synonymy: 1974: Vespertiliavus gracilis in Hartenberger et al., p. 193
1981: Vespertiliavus cf. gracilis (pro parte) in Crochet et al., tabl. 2-2
1987: Vespertiliavus gracilis in Remy et al., tabl. 1a p. 177
1988: Vespertiliavus gracilis in Sigé, p. 78–85, text-fig. 8–14
2006a: Vespertiliavus cf. gracilis in Maitre et al., p. 117, fig. 5a
2006: Vespertiliavus gracilis in Sigé & Crochet, p. 194
Diagnosis: (Partly based on Sigé’s (1988) description) Vespertiliavus of average dimensions (P/4–M/3: from 6.83 to 7.36 mm); pronounced postorbital processes; P/3 with two root sets obliquely with respect to the axis of the ramus. Vespertiliavus larger than V. gracilis; lower molars with trigonid more compressed and talonid wider than that of V. gracilis; C/1 wide and large; extended M3/ preparacrista.
Holotype: BRE_2-707, bony palate displaying alveoli for the right C1/–P2/ and left C1/P2–3/ and bearing the right P3–4/–M1/ and the left P4/–M1/ (p. 83 text-fig. 11, in Sigé [1988]), from the collections of UM2.
Type-locality: Le Bretou (MP 16), Tarn-et-Garonne, Phosphorites du Quercy.
Other localities: La Bouffie, St-Antonin-Noble-Val, Aubrelong 2, Les Pradigues, Clapassou (MP 17a), Bouyssou 2 (MP 18), Coânac 1 (MP 19).
Material and measurements: see Appendix 4.
Description: as in Sigé (1988). The morphological variation observed within the population of Le Bretou is also found at the other localities. On the upper molars, the posterior edge of the heel, usually straight, can be more rounded at some localities, the most lingual angle extending slightly posteriorly.
Comparison within the new subspecies V. disjunctus disjunctus: the specimens from the deposit at Les Pradigues are clearly larger than those found at Aubrelong 2, St-Antonin-N.-V., Clapassou, and la Bouffie.
Vespertiliavus disjunctus nauzensis nov. ssp. (Plate 12m–v)
Synonymy: 1987: Vespertiliavus sp. in Remy et al., tabl. 3a p. 183
Diagnosis: largest subspecies of V. disjunctus.
Derivatio nominis: from the name of the type-locality of this subspecies, La Nauze 1.
Holotype: TAB_VdnA.1.1, left M1/ (Plate 12t), from the UM2 collections.
Type-locality: Tabarly (MP 20), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Cloup d’Aural 1 (post MP 20), Souhic 1, Lapize (1, 2) (MP 21/22), La Nauze 1 (MP 22), Lébratières 15 (MP 22).
Material and measurements: see Appendix 4.
Description: the general morphology is identical to that of V. disjunctus disjunctus nov sp., nov. ssp.
Comparison of the new species V. disjunctus: this species is clearly smaller than V. schlosseri and larger than V. gracilis, from which it can be distinguished by the proportionally shorter P/4; lower molars with more transversely compressed trigonids. The talonid of M/1–2 is less reduced, unlike that of M/3. C1/ does not exhibit very pronounced posterolingual relief, and C/1 is clearly wider and larger. M3/ appears to be longer, due to the shorter preparacrista.
Its intermediate size clearly sets it apart from the larger forms of V. schlosseri, V. bourguignati and V. wingei, and the smaller V. gracilis and V. (Sigeia) lizierensis nov. sgen. nov. sp.
Subgenus Sigeia nov. sgen.
Diagnosis: all species of the genus Vespertiliavus with the reduced dental formula: I ?/2, C1/1, P 2?/2, M3/3. Nyctalodont to submyotodont lower molars; upper molars with hypocone.
Derivatio nominis: in honour of Dr. Bernard Sigé, who participated actively in collecting in the Quercy area for more than 40 years and is the author of numerous studies of fossil material from the Quercy, notably on insectivores and bats.
Type-species: V. (Si.) lizierensis nov. sp. Type-locality: St-Lizier (MP 16), Tarn, Phosphorites du Quercy, France.
Vespertiliavus (Sigeia) lizierensis nov. sgen., nov. sp. (Plate 13a–i)
Vespertiliavus (Sigeia) lizierensis nov. sgen., nov. sp. from Perrière, Les Pradigues, St-Lizier, La Bouffie and Aubrelong 2: a PRR_VlA.1.1, fragment of left hemimandible with I/1–2, C/1, P/2–4. b PRA_VlA.1.4, right M/1. c SLI_VlA.1.1, right M/2. d SLI_VlA.1.2, right M/3. e BFI_VlA.1.5, left C1/, labial view (left) and lingual view (right). f ABL2_VlA.1.1, left P4/. g SLI_VlA.2.5, right M1/. h SLI_VlA.2.3, holotype, left M2/. i SLI_VlA.2.7, right M3/. Vespertiliavus (Sigeia) recens nov. sgen., nov. sp. from Mas de Got: j MGT_VrA.1.5, fragment of left hemimandible with broken M/3. k MGT_VrA.1.4, right M/1. l MGT_VrA.1.3, right M1/. m MGT_VrA.1.1, holotype, left M2/. n MGT_VrA.1.2, left M3/
Synonymy: 1987: Vespertiliavus sp. in Remy et al., tabl. 1a p. 177
Diagnosis: the smallest species of this subgenus known to date; in regard to the species of the genus Vespertiliavus, its P/2 is much smaller than its P/4, and its M3/ has a very short preparacrista.
Derivatio nominis: from the name of the type-locality for this species, St-Lizier.
Holotype: SLIVlA.2.3, left M2/ (Plate 13h), from the UM2 collections.
Type-locality: St-Lizier (MP 16), Tarn, Phosphorites du Quercy, France.
Other localities: La Bouffie, Les Clapiès, Aubrelong 2, Les Pradigues (MP 17a), Perrière (MP 17b).
Material and measurements: see Appendix 4.
Description: I/1–2: two incisors observed in situ on a hemimandible fragment from Perrière (Fig. 18a).
Line drawings of the atypical characters of the dental morphology of Vespertiliavus (Si.) lizierensis nov. sp.: a two incisors and no P/3 (fragment left mand., PRR_VlA.1.1; labial view above, occlusal view in the middle, lingual view below); b lower molars nyctalodont (on the left; right M/2, SLI_VlA.1.1) to submyotodont (on the right; right M/1, PRA_VlA.1.5); c well-developed hypocone on upper molar (left M2/, SLI_VlA.2.3)
C/1 typical of the genus with a thin shape, a pronounced oblique cingulid only on the lingual face, widening anteriorly and slightly cuspidate at rear.
P/2: much smaller than P/4; uniradiculate; oval occlusal contour; protocone with two crests meeting the antero- and posterolingual corners; apex facing lingually.
P/3: completely absent.
P/4 much more developed than P/2; extended anteriorly; encircled by cingulid; two sinuous crests arising from protocone and meeting the antero- and posterolingual corners.
M/1–2 nyctalodont to submyotodont; typical morphology for the genus with a strong entoconid, a crestiform hypoconulid, a somewhat closed trigonid, extended transversely (Fig. 18b).
M/3 with somewhat closed trigonid, extended transversely; very squat talonid, with no hypoconulid.
C1/: typical of the genus; lingual cingulum disappearing at centre and strongly cuspidate posteriorly; high lamella.
P4/ typical of the genus with a dominant paracone bearing a prominent postparacrista, an anteriorly cuspidate parastyle, and a relatively well-developed heel.
M1/ with a well-defined labial edge: very deep indentation at the level of the paracone; very wide parastyle and antecingulum; prominent mesostyle on the labial edge; very open protofossa delimited by a very short postprotocrista, terminating in a projection; ectoloph with long branches; squat protocone and very wide protofossa; pronounced heel widening lingually to end in a slight point, with a wide cingulum bearing a distolingually cuspidate hypocone (not connected to the protocone) and a straight posterior edge; postcingulum connected to the heel cingulum.
M2/ of same morphology but with an anterior side as wide as the posterior side and much extended transversely (Fig. 18c).
M3/ with three ectoloph branches; antecingulum becoming thinner at the paracone but still connected to the preprotocrista.
Vespertiliavus (Sigeia) recens nov. sgen., nov. sp. (Plate 13j–n)
Diagnosis: clearly larger than V. (Si.) lizierensis; long lower molars and of submyotodont form; long upper molars with very indented labial edge.
Derivatio nominis: from the latin recens: recent, to highlight the fact that this new species V. (Si.) recens nov. sgen., nov. sp. is present in localities that are more recent than those where V. (Si.) lizierensis nov. sgen., nov. sp., a morphologically similar species, is found.
Holotype: MGTVrA.1.1, left M2/ (Plate 13m), from the collections of UM2.
Type-locality: Mas de Got (MP 22), Lot, Phosphorites du Quercy, France.
Material and measurements: see Appendix 4.
Description: M/1 submyotodont; strong entoconid; hypoconulid, small yet cuspidate.
M/3 with reduced talonid and no hypoconulid.
M1/ long, indented labial edge, strong parastyle, cuspidate metastyle; relatively squat protocone; short postprotocrista, leaving the protofossa wide open and ending in a projection; developed heel with cingulum connected to postprotocrista.
M2/: identical morphology to M1/ with the anterior side being the same width as the posterior side; thick cingulum on heel, with a clearly developed hypocone; somewhat straight posterior heel edge; hypocone separated from protocone by a vertical sinus (Fig. 19).
M3/ relatively long; with three ectoloph branches of which the third is almost as long as the second; pronounced parastyle; independent mesostyle, clearly offset posteriorly; postprotocrista separated from metastyle by a sinus.
Comparison: this species has a morphology very close to that of V. (Si.) lizierensis nov sgen., nov. sp. with many common features atypical for Vespertiliavus. Its upper molars have a cuspidate hypocone, a widely open protofossa due to a short postprotocrista and a squat protocone, and its lower molars exhibit a submyotodont structure. These common characteristics lead to the assumption of a simplified dental formula for V. (Si.) recens nov. sgen., nov. sp., in the same vein as V. (Si.) lizierensis nov. sgen., nov. sp., and thus of belonging to a subgeneric entity distinct from the other species of Vespertiliavus. V. (Si.) recens nov. sgen., nov. sp. can be distinguished from V. (Si.) lizierensis nov. sgen., nov. sp. by not only its larger size but also its longer upper molars with a more indented labial edge and the lower molars also being longer and with a more open trigonid.
Remarks on the subgenus Sigeia nov. sgen.: The specific characters displayed by the dentition of the two new species of Sigeia nov. sgen. appear atypical for fossil Emballonuridae, but are astonishingly similar to those in extant Emballonuridae, notably in the developed or at least incipient hypocone and the heel morphology of the upper molars.
Superfamily Rhinolophoidea BELL, 1836 (WEBER 1928)
Family Hipposideridae MILLER, 1907
“Like the Rhinolophidae, but pectoral and pelvic girdles more highly modified; toes with two phalanges each; and lumbar vertebrae showing a marked tendency to become fused into a solid rod. In the pectoral girdle the fusion of the first and second ribs involves the entire bone to and including the corresponding dorsal vertebrae. There is thus a solid ring of bone consisting of the seventh cervical vertebra, first and second dorsals, first and second ribs, and entire presternum, the elements of the ring indicated by a slit-like vacuity above, between the ribs and one or two small roundish vacuities below. Pelvic girdle like that of the Rhinolophidae posteriorly, but anteriorly with a supplemental bridge of bone connecting acicular process with front of ilium and producing a preacetabular foramen slightly exceeding the thyroid foramen in size” (Miller, 1907).
Current geographic distribution: Tropical parts of the Old World, east to the Philippine Islands, New Guinea, and Australia, extending to the edges of the tropical regions: Xiamen, Himalaya and Morocco.
Genus Hipposideros GRAY, 1821
Subgenus Pseudorhinolophus SCHLOSSER, 1887
Remarks: Schlosser (1887) included under this subgenus name several specimens which do not correspond to the genus Hipposideros, but rather to the genus Palaeophyllophora, notably in Plate II, figures 15, 24, 29, 33, 37. Revilliod, in his important study on the Quercy Chiroptera (Revilliod 1917), undertook a much more detailed characterization of this taxon based upon material that was, admittedly, more morphologically homogenous but of varied provenance and thus various ages. The current work helps to detail the characters connected to this variability and those related to the morphological evolution of this taxon over time.
Synonymy: 1872: Rhinolophus antiquus (pro parte) Filhol, p. 30, Pl. 19 fig. 47–50
Dental formula: I 1/2, C1/1, P2/2, M3/3
Amended diagnosis (Revilliod, 1917): Maxillary: M3/ quite reduced, nevertheless with crest posterior to mesostyle, of variable length, but most often not reaching half the length of the postparacrista.
M1–2/ of same length, the crests connecting the mesostyle to external cusps well developed; the anterior and posterior lobes are equal. The M/1 heel slightly better developed than that of M/2.
P1/: without labial cingulum.
P2/: always pushed to the exterior of the alveolar border, one root.
C1/: without labial cingulum; smooth anterior surface.
Hemimandible: talonid of M/1–2 well-developed width equal to that of trigonid; the posterolabial point barely reaches 2/3 of the height of the anterolabial point. M/3 talonid narrower than that of M/1–2, its three cusps clearly distinct from one another.
P/1 with rudimentary paraconid.
P/2 always absent.
P/3 longer than tall.
High coronoid process, not elongated. Angular process quite elongated but only very slightly inclined further on.
Type-species: Schlosser did not name a type-species or a species when he created genus Pseudorhinolophus. The species created by Pictet (1855), subsequently connected to this subgenus as H. (Ps.) morloti, was the first named and seems to represent the type-species.
Other species described: H.(Ps.) morloti morloti; H.(Ps.) morloti sequens nov. ssp.; H.(Ps.) weithoferi; H.(Ps.) schlosseri schlosseri; H.(Ps.) schlosseri salemensis nov. ssp.; H. (Ps.) zbrjdi; H. (Ps.) russelli nov. sp.; H. (Ps.) major nov. sp.; H. (Ps.) tenuis nov. sp.
Remarks on H. (Ps.) egerkingensis REVILLIOD, 1922: Observation of the three specimens from the Naturhistorisches Museum Basel (Ef. 995, 998, 994), as attributed by Revilliod (1922) to the genus Hipposideros, has revealed an error in determination. The maxillary Ef. 995 has a broken P4/ but exhibits a small protocone (small anterolingual swelling) connected to the paracone by an individualized crest. The M2/ has a very indented mesostyle, a large space between the preprotocrista, connected to the parastyle, and the base of the paracone, a posterolingually inflated heel, without distinct hypocone, and a sinuous labial edge; the M3/ exhibits a very labial parastyle, distant from the rest of the tooth. On the hemimandible Ef. 998, the alveoli indicate biradiculate P/3 and P/4. The only tooth present is a broken M/2 with base of the cusps rounded; the cingulids are pronounced and flanked by a marked sinus; the talonid is well developed and as long as the trigonid. The teeth of these two specimens are not like hipposiderids and appear to belong to the genus Stehlinia (Palaeochiropterygidae) and more precisely to the species S. gracilis.
Specimen Ef. 994 is a hemimandible displaying the alveolus of a vestigial P/3 and that of a uniradiculate P/2, and bears a much anteroposteriorly extended P/4. Thus, this specimen does not exhibit the characteristics of the subgenus Pseudorhinolophus or the genus Stehlinia as do other specimens connected to H. (Ps.) egerkingensis. The morphology exhibited by P/4 seems to bring this specimen closer in line with species of Carcinipteryx (see below), despite the presence of P/3.
Distribution: from the upper basal Eocene (MP 16) to the upper basal Oligocene (MP 25) in western Europe (France and part of Switzerland).
Hipposideros (Pseudorhinolophus) morloti (PICTET, 1855)
Remarks: within the species H. (Ps.) morloti, two subspecies can be distinguished essentially by their sizes and certain morphological traits. De Bonis et al. (1973) separated these two subspecies by noting Hipposideros (Ps.) morloti 1 for the localities of Escamps, Rosières 1 and Rosières 4, and H. (Ps.) morloti 2 for Aubrelong 1, Ravet déblais and Mas de Got. These subspecies changes seem to appear around reference level MP 20/21 (Coyrou 1-2).
Synonymy: 1855: Vespertilio morloti in Pictet et al., p. 76, Pl. 6 fig. 1–6
1869: Vespertilio morloti in Pictet & Humbert, p. 127, Pl. 14 fig. 1
1887: Pseudorhinolophus morloti in Schlosser, p. 69
1891: Pseudorhinolophus morloti in Zittel, p. 576
1896: Pseudorhinolophus morloti in Roger, p. 28
1898: Pseudorhinolophus morloti in Trouessart, p. 95
1904: Pseudorhinolophus morloti in Trouessart, p. 69
1967: Pseudorhinolophus aff. morloti in Miguet, p. 106–108
1968: Hipposideros (Pseudorhinolophus) morloti in Sigé, p. 99
1973: Hipposideros (Ps.) morloti 1 et 2 in de Bonis et al., tabl. 2a
1979: Hipposideros (Pseudorhinolophus) morloti and Hipposideros (Pseudorhinolophus) sp. in Sigé et al., p. 46 and 87
1981: Hipposideros (Ps.) morloti and cf. in Crochet et al., tabl. 2-2
1987: Hipposideros (Ps.) cf. morloti in Remy et al., tabl. 1a-3a, p. 177–183
2006a: Hipposideros (Ps.) morloti in Maitre et al., p. 117, fig. 5a
Diagnosis: same as that of subspecies H. (Ps.) morloti morloti (see below).
Hipposideros (Pseudorhinolophus) morloti morloti (PICTET 1855) (Plate 14a–e)
Hipposideros (Pseudorhinolophus) morloti morloti (Pictet 1855) de Salème: a SA_HpmmF.1.4, right C/1, labial view (left) and lingual view (right). b SA_HpmmA.1.7, fragment of left hemimandible with P/2–4 and broken M/1–2. c SA_HpmmA.1.15, fragment of left hemimandible with M/2–3. d SA_HpmmE.1.2, left C1/, labial view (left) and lingual view (right). e SA_HpmmC.1.11, fragment of left maxillary with P4/–M1–2–3/. Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp. From Aubrelong 1 and Gardiol: f ABL1_HpmsA.2.16, left C/1, labial view (left) and lingual view (right). g GAR3_HpmsA.1.30, fragment of right hemimandible with alveoli for P/2 et M/1, and with P/4. h GAR3_HpmsA.1.11, right M/1. i GAR3_HpmsA.1.1, right M/2. j GAR3_HpmsA.1.23, right M/3. k GAR3_HpmsA.4.19, right C1/, labial view (left) and lingual view (right). l GAR3_HpmsA.3.20, right P4/. m GAR3_HpmsA.2.10, right M1/. n GAR3_HpmsA.2.16, holotype, right M2/. o GAR3_HpmsA.2.3, left M3/
Diagnosis (the original author provides almost no detail on this taxon but the present work offers a proposal based upon material that is more distinctly representative): species of intermediate size between H. (Ps.) tenuis and H. (Ps.) schlosseri. Lower and upper molars and P4/4 are respectively wider than those of previous species.
Derivatio nominis: nominal subspecies.
Lectotype: Pictet-featured four unlisted hemimandibles, amongst them that in his figure 4 of plate 6 which is the best preserved. It is proposed here that this specimen be now considered the lectotype of this subspecies, given that it provides a good representation of the taxon; from the collections of the Naturhistorisches Museum Basel.
Type-locality: Saint-Loup du Mormont, Switzerland. Reference population: Salème (MP 17a), Lot, Phosphorites du Quercy, France.
Other localities: St-Lizier, Le Bretou, Lavergne (MP 16), Lébratières 1, Ginouillac, La Bouffie, La Cantine 2, Aubrelong 2, Trifon, Les Pradigues (MP 17a), Coustal (MP 17a/b), Perriqre, Rosiqres 5, Ppppnut, Sorciqres, Pech d’Isabeau (MP 17b), Bouyssou 2 (MP 18), Lostange (MP 19).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus approximately as tall as one dental crown, and mental foramen beneath the front of P/2; subvertical ascending ramus, with base elevated with respect to horizontal ramus; deep masseteric fossa extending to the upper edge of the ascending ramus; thick anterior and lower edges; axis formed by the condyle and the anterior edge of the ascending ramus, slightly more lateral than mandibular axis (Fig. 20a).
Line drawings of the hemimandibles observed for the species of the genus Hipposideros (Pseudorhinolophus): a H. (Ps.) morloti morloti (SA_HpmmB.3.22); b H. (Ps.) schlosseri schlosseri (SNB_51.4); c H. (Ps.) schlosseri salemensis nov. ssp. (PRA_HpmsA.1.1); d H. (Ps.) russelli sp. nov. (TRI_HprAD.4.1, inverted); e H. (Ps.) major nov. sp. (ROS1_HpdB.3.14, inverted); f H. (Ps.) tenuis nov. sp. (LOS_HptA.1.1, inverted)
Two incisors indicated by alveoli anterior to C/1 but not observed.
C/1 typical of the genus; convex labial aspect with a slight cingulid at the base; convex lingual face, rather flat posteriorly and with a pronounced cingulid at base, wide anteriorly and posteriorly and posteriorly cuspidate; edge of the crown slightly raised anteriorly; apex of tooth lingually orientated.
P/2 uniradiculate; oval occlusal contour; composed of a dominant protoconid, lingually orientated and with a pre- and postprotocristid connecting the slightly pinched anterior and posterior extremities.
P/4 with a protoconid and a rudimentary metaconid; widened anterolingual and posterior corners; coronal edge sinuous on the labial side and oblique on the posterior side (rising lingually); cingulid surrounding tooth, except at the base of the metaconid, cuspidate at the anterolingual corner and posteriorly to the base of the metaconid.
M/1–2 nyctalodont, with consistent hypoconulid; talonid barely offset labially in relation to the trigonid; M/1 different from M/2 due to a more open, narrower trigonid.
M/3 slightly smaller than M/2 with a more enclosed trigonid and a reduced talonid.
C1/: labial cingulum not distinct, lingual cingulum anteriorly thick and ending in projection on labial side, widened posteriorly; small swelling sometimes cuspidate at the base of the posterior edge of the tooth, at the junction of the flat lingual face and convex labial aspect.
P2/ unicuspidate, revealed by its alveolus adpressed against the most anterior root of P4/. P4/ practically rectangular, slightly anteroposteriorly pinched; dominant paracone with prominent postparacrista, ante- and postcingulum; cuspidate protocone, well-developed heel with a basin.
M1–2/: open protofossa on M1/ and closed on M2/; internal ectoloph branches as long as the external branches, so that the mesostyle projects from the labial edge of the tooth; strongly developed heel, extending posteriorly mainly on M1/, and with a thick cingulum connected by a slight crest to the projection of the postprotocrista.
M3/ slightly reduced, with three ectoloph branches, of which the most posterior is the shortest, extended protofossa, cingulum connecting the parastyle to the metacone passing through the protocone; distinct metaconule.
Comparison within the subspecies H. (Ps.) morloti morloti: the population of Le Bretou shows specimens slightly smaller than those of Lavergne and larger than those of St-Lizier. The material from Les Pradigues is still larger than that of La Bouffie. At reference level MP 17b, the specimens from the Sorcières deposit seem to be slightly larger than those from Rosières 5.
Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp. (Plate 14f–o)
Diagnosis: subspecies distinct from H.(Ps.) morloti morloti due to slightly larger size, an open protofossa on M2/ and upper teeth with heel generally less extended posteriorly.
Derivatio nominis: from the Latin sequens: successor, because of having morphology similar to that of the other subspecies occurring in the older localities.
Holotype: GAR3HpmsA.2.16, right M2/ (Plate 14n), from the collections of UM2.
Type-locality: Gardiol 3 (MP 23), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Coyrou 1-2 (MP 20/21), Lébratières 2 (post MP 20), Aubrelong 1 (MP 21), Lapize (1, 2) (MP 21/22), La Couaille, La Plante 2, Mas de Got, Jammes, Baraval, Cavalé, Lébratières 12, 13, 15, Pendaré (MP 22), Gardiol 3 (MP 23).
Material and measurements: see Appendix 4.
Description: the general morphology is identical to that described for H. (Ps.) morloti morloti; the few observable morphological differences are those listed in the diagnosis (Fig. 21). Comparison within the subspecies H. (Ps.) morloti sequens: the relative sizes of the specimens show that those from La Plante 2 are slightly smaller than those from Mas de Got. Comparison of H. (Ps.) morloti with the other species of the genus: these two subspecies are clearly distinct from H. (Ps.) schlosseri, being of smaller size, with wider P4/4, and a heel on M1/ that reaches further posteriorly. They are also distinct from H. (Ps.) tenuis nov. sp., being larger, with less elongated and proportionally larger lower teeth, noticeably wider upper molars with a posteriorly extended heel.
Comparison of two subspecies of H. (Ps.) morloti: a heel more posteriorly extended on M1/ (right M1/, SAL_HpmmC.1.5) and b closed protofossa on M2/ (left M2/, inverted, SAL_HpmmC.3.26) of H. (Ps.) morloti morloti in contrast to those of H. Ps. morloti sequens (c left M1/, inverted, ABL1_HpmpA.3.5; d right M2/, ABL1_HpmpA.3.3)
Hipposideros (Pseudorhinolophus) weithoferi REVILLIOD, 1917
Synonymy: 1967: Pseudorhinolophus weithoferi in Miguet, p. 108–109
1968: Hipposideros (Pseudorhinolophus) weithoferi in Sigé, p. 99
1979: Hipposideros (Pseudorhinolophus) weithoferi in Sigé et al., p. 47, 92
Type: Revilliod having featured two hemimandibles and a maxillary, the choice made here is to regard hemimandible Q.P. 804, the right hemimandible displaying the alveoli for I/12, C/1 and P/2, and bearing P/4–M/1–2–3, as the lectotype for this species; figured in Revilliod 1917, Fig. 4 p. 13, from the collections of the Naturhistorisches Museum Basel.
Type-locality: Old Quercy Collections (unknown locality, indeterminate age).
Measurements: see Appendix 4.
Remarks: very large specimens (size range of Palaeophyllophora oltina), larger than H. (Ps.) major. M/1 with narrow trigonid.
Hipposideros (Pseudorhinolophus) schlosseri Revilliod 1917
Remarks: study of all the specimens connected to H. (Ps.) schlosseri reveals modifications (reduction of P2/2 and increase in molar size) starting from reference level MP18. These changes are not significant enough to distinguish two species but nevertheless allow the distinction of two chronological subspecies.
Synonymy: 1968: Hipposideros (Pseudorhinoloplus) schlosseri in Sigé, p. 99
1973: Hipposideros (Ps.) cf. schlosseri in de Bonis et al., tabl. 2a
1978: Hipposideros (Ps.) schlosseri in Sigé, p. 251–254, Pl. 1 fig. 1–2, 7
1979: Hipposideros (Pseudorhinoloplus) schlosseri in Sigé et al., p. 47, 92
1981: Hipposideros (Ps.) schlosseri et cf. in Crochet et al., tabl. 2-2
1987: Hipposideros (Ps.) schlosseri et cf. in Remy et al., tabl. 1a-3a p. 177–183
1988: Hipposideros (Ps.) schlosseri in Sigé, p. 89–92
1995: Hipposideros (Ps.) cf. schlosseri in Legendre et al., p. 64–65
2006a: Hipposideros (Ps.) schlosseri in Maitre et al., p. 116–118, fig. 5a
2006: Hipposideros (Ps.) schlosseri in Sigé & Crochet, p. 199
Amended diagnosis (the author does not strictly speaking provide a diagnosis, but this work, based on much more material, allows one to be proposed), i.e. that of H. (Ps.) schlosseri schlosseri.
Hipposideros (Pseudorhinolophus) schlosseri schlosseri REVILLIOD, 1917
Diagnosis: larger species than H. (Ps.) morloti; smaller and with less reduced M3/3 than H. (Ps.) russelli.
Derivatio nominis: nominal subspecies.
Lectotype: Revilliod (1917) having failed to assign a type, Sigé (1978) proposed the most complete specimen, Q.P. 875, a right maxillary bearing C1/–M3/, fig. 7, p. 15, Revilliod 1917, to be considered the lectotype for this species; from the collections of the Naturhistorisches Museum Basel.
Type-locality: Old Quercy Collections (unknown locality, indeterminate age), France.
Reference population: Ste Néboule (MP 18), Lot, Phosphorites du Quercy, France.
Other localities: Bouyssou 2, Théron, Crégols, Monteils, Mas de Labat 1, Gousnat, Sindou D (MP 18), Les Trémouls (MP 18/19), Guirolle rouge, Rosières 1, 2, 4, Coânac 1, Escamps, Nougayrac, Palembert, Célarié ocre, Célarié standard, Lostange, (MP 19), Pécarel, Tabarly (MP 20), Coyrou 1-2 (MP 20/21), Ravet déblais, Escabasse 2 (MP 21), Souhic 1 (MP 21/22), La Plante 2 (MP 22).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus approximately as deep as a dental crown; ascending ramus rising quickly posterior to M/3, low, with the base strongly oblique with respect to the horizontal axis and the pseudo-vertical upper section; masseteric fossa extended to the upper part of the ascending ramus; condyle better developed on lateral side; longitudinal internal crest crossing the ascending ramus at the maximum height of the teeth; angular apophysis clearly directed ventrally (Fig. 2a, b).
Two lower incisors, most often indicated by their alveoli.
C/1 composed of three aspects: flat anterolingual and posterolingual faces with a mostly horizontal base and a convex lingual surface; surrounding cingulid, very thick both lingually and posteriorly and rising slightly anteriorly; apex directed lingually.
P/2 unicuspidate, with oval occlusal contour, composed of a protoconid with pre- and postprotocristae meeting the angular extremities of the tooth.
P/4 biradiculate, with trapezoidal occlusal contour (anterior edge shorter than posterior edge); dominant protoconid; slightly cuspidate metaconid; enlarged, cuspidate anterolingual angle; posterior angle strongly enlarged; cingulid all around except at base of metaconid, slightly raised posteriorly and labially sinuous.
M/1–2 nyctalodont; long pre-entocristid; M/1 trigonid more open and narrower than that of M/2.
M/3 smaller than M/2; reduced talonid with entoconid, hypoconid and hypoconulid.
C1/: thick lamella with flat lingual face and convex labial surface, separated by two sharp edges, the posterior aspect with a relief as 1/3 height of the dental crown; ridge only on the lingual edge, indented or sinuous, terminating in a small posterior swelling and anterior projection, as slight anterior rise of the edge of the crown; apex facing posteriorly.
P2/ unicuspidate, stylet shaped.
P4/ with quasi-rectangular occlusal contour, sometimes anteroposteriorly pinched on the posterior edge; dominant paracone with prominent postparacrista; pronounced surrounding cingulum except on labial side; protocone more or less cuspidate, and heel more or less developed posteriorly, potentially bearing a basin.
M1/ square and M2/ rectangular; internal ectoloph branches equal in length to external branches, such that the mesostyle projects from the labial edge; protofossa open posteriorly on M1/ and closed on M2/, either due to the junction between postprotocrista and basis of the metacone or the junction between postprotocrista and postcingulum; pronounced parastyle; heel more developed on M1/ than on M2/, somewhat extended posteriorly; thick heel cingulum connected to postcingulum.
M3/ with three ectoloph branches; pronounced parastyle; developed protocone; extended protofossa, of variable length.
Comparison within the subspecies H. (Ps.) schlosseri schlosseri: observation reveals an increase in size of specimens ranging from the Gousnat population to that of Sindou D, then Ste-Néboule, for deposits from reference level MP 18. At MP 19, size increases from Rosières 4, to Rosières 2, then Coânac 1, Rosières 1, and finally Célarié ocre.
Hipposideros (Pseudorhinolophus) schlosseri salemensis nov. ssp. (Fig. 20; Plate 15)
Hipposideros (Pseudorhinolophus) schlosseri salemensis nov. ssp. from Salème and Bretou: a SA_HpssA.2.1, right hemimandible, labial view (top), occlusal view (centre), and lingual view (bottom), with alveoli for I/1–2, and with C/1, P/2–4, M/1–2–3. b BRE_2-857, right C1/ labial view (left) and lingual view (right). c SA_HpssA.1.1, holotype, fragment of right maxillary droit with alveolus for P2/, and with P4/ and M1–2–3/
Diagnosis: smallest subspecies of H. (Ps.) schlosseri, and with proportionally reduced P2/2.
Derivatio nominis: from the name of the type-locality of this subspecies, Salème.
Holotype: SAHpssA.1.1, left P4/–M3/ (Plate 15c), from the UM2 collections
Type-locality: Salème (MP 17a), Lot, Phosphorites du Quercy, France.
Other localities: St-Lizier, Le Bretou (MP 16), Glaudys (Eocène supérieur indet.), Tufal, La Bouffie, St-Antonin-Noble-Val, Aubrelong 2, Les Pradigues, Bouziès (MP 17a), Perrière, Rosières 5, Malpérié, Pépénut, Bouyssou 3 (MP 17b).
Material and measurements: see Appendix 4.
Description: the general morphology is identical to that of H. (Ps.) schlosseri schlosseri; the few observable morphological modifications are those noted in the diagnosis (Fig. 22).
Comparison of the species H. (Ps.) schlosseri: some variability exists within this species. The population from La Bouffie has upper molars that are wider (transversely developed). The opening of the protofossa for M1/ does not appear to be generalized in all other localities. At Coyrou 3, for example, the majority of specimens have a closed protofossa; at Malpérié, the two morphotypes are present in equivalent proportions.
H. (Ps.) schlosseri is of intermediate size between H. (Ps.) morloti and H. (Ps.) russelli nov. sp. It can be distinguished from the former of these species by the proportionally longer P4/4 and a less extended M1/ heel. It can be distinguished from the latter species by its less reduced M3/3.
Hipposideros (Peudorhinolophus) zbrjdi SIGÉ, 1990
Previous references: 1995: Hipposideros (Ps.) zbrjdi in Sigé, p. 100–105, text-fig. 24–29
2006: Hipposideros (Ps.) zbrjdi in Sigé & Crochet, p. 200
Original diagnosis: small-sized Pseudorhinolophus; moderate reduction of M3/3; advanced reduction of the vestigial P/2; presence of a distal fold on the protocone of M1/. Type: (from the collections of UM2) BEL 1165.1, a well-preserved left maxillary fragment bearing C1/–M3/.
Type-locality: Belgarric (MP 25), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Le Garouillas (faciqs indurp), La Garrigue, L’Escoufle, Ppcofi, Rigal-Jouet (MP 25).
Material and measurements: see Appendix 4.
Remark: no new material is assigned to this Pseudorhinolophus small species. The description and comparison of the different dental categories and of the mandible are given by Sigé (1995).
Hipposideros (Pseudorhinolophus) russelli nov. sp. (Plate 16)
Hipposideros (Pseudorhinolophus) russelli nov. sp. from Trifon: a TRI_HprE.2.2, right C/1, labial view (left) and lingual view (right). b TRI_HprB.2.9, right P/2. c TRI_HprB.3.10, right P/4. d TRI_HprB.1.1, left hemimandible with alveoli for C/1, P/2–4, and with M/1–2–3. e TRI_HprE.1.9, left C1/, labial view (left) and lingual view (right). f TRI_HprD.1.1, holotype, fragment of right maxillary with alveoli for C1/ et P2/, and with P4/ and M1–2–3/
Synonymy: 1978: Palaeophyllophora sp. in Sigé, p. 261
1987: Hipposideros (Ps.) sp. in Remy et al., tabl. 1a, p. 177
2000: Hipposideros (Ps.) cf. schlosseri in Astruc et al., table 1, p. 278
2006a: Hipposideros (Ps.) sp. A and B, and H. (Ps.) schlosseri (pro parte) in Maitre et al., p. 116–117, fig. 3a, b and 5a
Diagnosis: species of larger size than that of H. (Ps.) schlosseri, distinct because of M/3 with more reduced talonid and shorter M3/, where the third ectoloph branch is shorter.
Derivatio nominis: in honour of Donald E. Russell, who contributed to a better understanding of the bats of the lower and middle Eocene, and of the origins of this highly diversified group.
Holotype: TRI Hpr D.1.1, right maxilla displaying alveoli of C1/ and P2/, and bearing P4/M1–2–3/ (Plate 16f), from the UM2 collections.
Type-locality: Trifon (MP 17a), Lot, Phosphorites du Quercy, France.
Other localities: Salème, Les Clapiès, La Cantine 2, Aubrelong 2, Bouziès, Clapassou (MP 17a), Coyrou 3, Sorcières (MP 17b), Mémerlin-Muséum, Monteils, Mas de Labat 1, Sindou D (MP 18).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus as deep as a dental crown height; long condyle; base of the ascending ramus barely elevated above the horizontal (Fig. 20d).
Two alveoli are the only indication of the existence of two lower incisors anterior to C/1.
C/1 apex directed slightly posterolingually, with three aspects: a labial convex surface and flat anterolingual and posterolingual faces; slight surrounding cingulid, oblique (slightly raised anteriorly), enlarged on the lingual edge, and even more so on the posterior edge, ending in slight point at the posterolingual corner.
P/2 unicuspidate; clearly smaller than P/4; oval occlusal contour, slightly pinched at the anterior and posterior extremities; protoconid with pre- and postprotocristae.
P/4: anterior edge shorter than posterior edge; slight sinuous cingulid lingually and labially; dominant protoconid; rudimentary metaconid; slight transversal postprotoconid basin; three aspects: a convex labial aspect, and flat anterolingual and posterolingual faces.
M/1–2: nyctalodont structure with hypoconulid slightly jutting posteriorly; talonid a little offset labially with respect to the trigonid, but of same width; trigonid more open and narrower on M/1; entoconid and hypoconulid of same volume.
M/3: talonid reduced in comparison to that of M/2; small entoconid and hypoconulid.
C1/ composed of two aspects: one flat lingual face and one convex labial surface; apex directed slightly lingually; lingual cingulum, with pointed swelling at posterior extremity, becoming wider anteriorly where it ends in a projection; sinuous labial crown edge, raised both anteriorly and posteriorly.
P2/ vestigial, stylet shaped; located on the labial edge of the maxilla.
P4/ clearly rectangular; dominant paracone; cuspidate protocone; heel, well developed posterolingually and surrounded by a cingulum; pronounced antecingulum terminating abruptly at base of paracone; rather short postparacrista; mild anteroposterior pinching of the posterior edge.
M1/ square shaped, M2/ more transverse; internal ectoloph branches of same length as external ones; mesostyle on labial edge, protruding sometimes more labially; closed protofossa; strong parastyle; heel developed on M1/, somewhat extended posteriorly and bearing thick cingulum.
M3/ reduced; three ectoloph branches, the posterior being the shortest; relatively extended protofossa; protocone connected to parastyle and pseudometacone; variable presence of small metaconule, more or less distinct.
Comparison: in comparison to H. (Ps.) major nov. sp., H. (Ps.) russelli nov. sp. is smaller, P/2 clearly bigger, P/4 and trigonid of M/3 wider, P4/ proportionally more transverse and M3/ with a longer third ectoloph branch. H. (Ps.) russelli nov. sp. is larger than H. (Ps.) schlosseri and can be otherwise distinguished morphologically by the more reduced M/3 talonid, and M3/ being more transverse, with a shorter third ectoloph branch.
Hipposideros (Pseudorhinolophus) major nov. sp. (Plate 17)
Hipposideros (Pseudorhinolophus) major nov. sp. from Rosières 1: a ROS1_HpmaB.1.8, left hemimandible, labial view (top), occlusal view (centre), lingual view (bottom), with alveoli for I/1–2, and with C/1, P/2–4 and M/1–2–3. b ROS1_HpmaA.3.3, fragment of right maxillary with C1/, P2–4/ and M1–2/: labial view of C1/ (right), and occlusal view of toothrow (left). c ROS1_HpmaA.3.9, holotype, fragment of left maxillary with alveoli for P4/, and with M1–2–3/
Synonymy: 1998: Hipposideros (Pseudorhinolophus) schlosseri in Sigé et al., p. 87
Diagnosis: H. (Pseudorhinolophus) larger than H. (Ps.) russelli and can be distinguished by: proportionally narrower P4/4; very reduced M/3 talonid; open M1/ protofossa; very short third ectoloph branch on M3/.
Derivatio nominis: from the Latin major: the biggest, due to its large size.
Holotype: ROS1HpmaA.3.9, fragment of right maxilla bearing M1–3/, (Plate 17c), from the UM2 collections.
Type-locality: Rosières 1 (MP 19), Lot, Phosphorites du Quercy, France.
Other localities: Ste-Néboule (MP 18), Rosières 3, Rosières 4 (MP 19), Tabarly (MP 20), Mas de Labat 2 (MP 21), Lapize (1, 2), La Rode, Pech Pulle (MP 21/22) Baraval, Lébratières 12, Lébratières 15 (MP 22).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus shallower than one molar crown and ascending ramus approximately twice that height; masseteric fossa slightly elevated posteriorly; angular apophysis extending posteriorly; upper part of the ascending ramus almost vertical; base of hemimandible concave ventrally from M/3 to posterior extremity (Fig. 20e).
I/1–2: presence indicated by alveoli anterior to C/1.
C/1 apex directed posterolingually; three aspects: a convex labial surface and flat anterolingual and posterolingual faces; cingulid raised (high/arising) anteriorly, enlarged (thickened) mainly on the posterior margin and ending in a posterolingual corner.
P/2: unicuspidate, with oval occlusal contour; cusp with pre- and postcristid.
P/4 larger than P/2; anterior edge shorter than posterior edge, with cingulid becoming very sinuous labially; dominant protoconid, flanked by a sinus on anterolingual and posterior sides; slightly cuspidate metaconid.
M/1–2 nyctalodont; hypoconulid slightly prominent posterolingually; talonid with slight labial offset in comparison to trigonid, but of same width; trigonid more open on M/1 and less so on M/2.
M/3 smaller than M/2; very reduced talonid; small entoconid and hypoconulid (Fig. 23a). C1/ slightly facing lingually (or tip directed lingually); with flat lingual face and convex labial aspect; lingual cingulum only, progressively widening anteriorly before disappearing labially, with a small swelling at posterior extremity; labial edge of the tooth raised at front and back.
P2/ vestigial, on the labial edge of maxilla.
P4/ rectangular, dominant paracone with cuspidate postparacrista, protocone and parastyle, connected by a precingulum; very posterolingual heel, extended and flanked by a crest.
M1/ square, M2/ more transversely developed; internal ectoloph branch of same length as external branches and mesostyle projecting onto the labial edge of the tooth; protofossa can be posteriorly open; strong parastyle; strong heel on M1/ and more moderate on M2/, with a large crest.
M3/ reduced, three ectoloph branches, the most posterior very short; reduced protofossa and protocone connected to parastyle and extremity of the third crest (Fig. 23b).
Comparison: the Rosières 1 population specimens are smaller than other populations of H. (Ps.) major, but the well-preserved specimens illustrate the specific characteristics of this taxon very well.
H. (Ps.) major nov. sp. is larger than H. (Ps.) russelli nov. sp. P/2 is smaller, P/4 and the trigonid of M/3 are narrower, P4/ is less transversely extended, and M3/ has a much reduced third ectoloph branch.
Hipposideros (Pseudorhinolophus) tenuis nov. sp. (Plate 18a–f)
Hipposideros (Pseudorhinolophus) tenuis nov. sp. from Lostange, Escamps and St-Lizier: a ESCA_HptA.1.4, right C/1, labial view (left) and lingual view (right). b LOS_HptA.1.1, holotype, left hemimandible with alveoli for I/1–2, C/1 and P/2, and with P/4 et M/1–2–3. c SLI_HptA.1.1, broken right C1/, labial view (left) and lingual view (right). d ESCC_HptA.1.4, right maxillary with P4/–M1/. e LOS_HptA.1.8, left maxillary gauche with alveoli for P2–4/ et*** M3/, and with M1–2/. f ESCA_HptA.1.1, right M3/. Hipposideros indet. from Chamblon: g CHA_HspA.4.7, broken right M/2. h CHA_HspA.4.8, talonid of broken left M/3. i CHA_HspA.1.6, right C1/, labial view (left) and lingual view (right)
Synonymy: 1987: Hipposideros (Ps.) sp. in Remy et al., tabl. 2a, p. 180
Diagnosis: smallest known species of this genus and morphologically distinct from H. (Ps.) morloti by the generally longer and narrower teeth; P4/ with less developed heel and less distinct cingulum; upper molars with a more lingual heel.
Derivatio nominis: from the Latin tenuis: slender, fine, thin, in reference to its very small size.
Holotype: LOS HptA.1.1, hemimandible displaying alveoli of I/1–P/2 and bearing P/4–M/3, (Plate 18b), from the UM2 collections.
Type-locality: Lostange (MP 19), Lot, Phosphorites du Quercy, France.
Other localities: St-Lizier, Le Bretou (MP 16), Glaudys (Eo. sup. indet), La Bouffie (MP 17a), Coyrou 3 (MP 17b), Sindou D (MP 18), Rosières 1, 2, 4, Escamps, Nougayrac, Guirolle blanc (MP 19).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus height equivalent to that of one molar crown; base of ascending ramus very elevated; angular apophysis very wide; ascending ramus vertical and low; condyle better developed laterally (Fig. 20f). Sharp teeth.
In all likelihood: two lower incisors, as demonstrated by two alveoli anterior to C/1. C/1 bearing slightly sinuous cingulid labially, more pronounced on lingual face and anteriorly thickened; small posterior swelling cuspidate at the base of the lamella; very flat lingual and convex external aspects.
P/2 never observed in situ; indicated by the elongated alveolus anterior to P/4.
P/4: occlusal contour ending in a point anteriorly, and a transverse edge posteriorly; tooth composed of a dominant protoconid and three sharp crests separating the flat anterolabial face from the concave anterolingual and posterior aspects; raised posterolingual corner.
M/1–2 long; M/1 trigonid more open and anteriorly extended than on M/2; nyctalodont form; hypoconulid very close to entoconid; M/2 talonid wider than that of M/1.
M/3 smaller than M/2; slightly reduced talonid.
C1/: sinuous labial edge rising anteriorly; lingual cingulum thick and wide, ending anteriorly in a projection labially; posteriorly directed apex.
P2/ uniradiculate; never observed in situ.
P4/ with a dominant paracone and a clearly cuspidate protocone; well-developed heel with cingulum; occlusal contour anteroposteriorly pinched.
M1–2/ with protofossa possibly open on M1/ and closed on M2/; heel developed and more lingually extended on M1/ than on M2/, tendency to exhibit a nascent hypocone; internal ectoloph branches as long as external ones; mesostyle clearly protruding beyond the labial edge of the tooth.
M3/ long, with three ectoloph branches; extended protofossa; protocone connected to parastyle and metacone.
Comparison: H. (Ps.) tenuis nov. sp. is clearly smaller than the other known species of this genus. It can be distinguished from H. (Ps.) morloti by its longer and narrower teeth, more lingually directed heel on upper molars, the less developed heel on P4/ and the less clearly defined cingula. Also distinguished from H. (Ps.) schlosseri by teeth (P/4 and M/1–3) that are more anteriorly extended, trigonid more open, M/3 talonid shorter, the hypoconulid closer to the entoconid, P4/ proportionally narrower and without cingulum, and M3/ with parastyle extended more labially, and a precingulum that is better separated from the flank of the paracone.
Hipposideros indet. (Plate 18g–i)
Locality: Chamblon (MP 13), Switzerland.
Material and measurements: see Appendix 4.
Description: there are a few teeth (2 C1/, 1 broken M/2 and 1 talonid of M/3?), which exhibit characteristics apparently closer to those of Hipposideros.
C1/, of which one is bigger, perhaps suggestive of sexual dimorphism; not thick, not tall and with a very thin apex; slightly sinuous coronal base on the labial side (convex aspect); curved lingual surface, separated by a large fossa from a basal cingulum becoming thicker anteriorly, and labially forming a projection.
M/2 nyctalodont; rather short, with a trigonid as wide as the talonid; relatively pronounced entoconid, developed precingulid.
M/3: talonid significantly narrower, composed of three small cusps; entoconid in anterior position and clearly separate from the hypoconulid.
Remarks: The occurrence of this unique taxon being the earliest of its type in western Europe, comparisons are limited. All the characters here described indicate referral to the genus Hipposideros, but the small number of specimens does not allow further determination.
Genus Palaeophyllophora REVILLIOD, 1922
Synonymy: 1872: Rhinolophus antiquus (pro parte) in Filhol, p. 30, Pl. 19 fig. 47–50
1922: Paraphyllophora in Revilliod, p. 160–162, fig. 65–67 and Pl. 4, fig. 12–14
1979: Paraphyllophora robusta in Sigé et al., p. 48, 92
Dental formula: I 1/2, C1/1, P2/2 or 3, M3/3
Original diagnosis: M3/ very reduced, no rudimentary crest posterior to mesostyle (as in Recent H. commersoni, Asellia tridens, Megadermatidae). M1/ and M2/: postparacrista and premetacrista much reduced. Anterior lobe less developed than posterior lobe. P1/: with well developed labial cingulum. P2/ most often with two roots (sometimes one forked root).
C1/ with labial cingulum, its anterior face marked by longitudinal groove. M/1 and M/2 characterized by talonid shorter than trigonid, also narrower. M/3: elongated and narrow talonid; internal point joined at the posterior extremity with the external point. P/2 rudimentary; always present, extruded from tooth row on alveolar border. Mandible: coronoid process well developed; square angular process; sinuous ventral margin of hemimandible.
Amended diagnosis: large teeth; necromantodont lower molars (with median hypoconulid); slight longitudinal groove on C1/.
Type-species: Revilliod (1917) did not designate a type-species when erecting this genus. Instead, he first created two species distinguishable by size, P. sanctae-neboulae and P. quercyi, then, in 1922, returned to the denomination of the first which he recognized as being synonymous with Vespertilio oltina Delfortrie 1873, based upon similarities in size, morphology and a conclusion as to the proximity of their provenance, Ste-Néboule de Béduer (Quercy). Priority rules indicate that P. oltina corresponds to the type-species for this genus.
Other species described: P. quercyi; P. nova nov. sp.; P. parva nov. sp.; P. rosierensis nov. sp.; ? P. sp.
Distribution: from the middle Eocene (MP 13?) to lower Oligocene (MP 23) in western Europe (France).
Discussion on the genus Paraphyllophora REVILLIOD, 1922:
In 1922, Revilliod proposed a new genus of Hipposideridae, close to the genus Palaeophyllophora but differing in the lack of P/3, being smaller than P. quercyi, the different shape of the cerebral cranium, a more defined mesostyle on the upper molars, a reduced talonid on the lower molars, and finally a third ectoloph branch on M3/. Based on observation of the material from the many and varied Quercy localities now known, this genus can be invalidated. Several of these diagnostic characters have been shown to be intraspecifically variable, notably the presence of P/3 in specimens of P. quercyi (for the most part, and also in the material of P. rosierensis nov. sp. from Escamps), with localities where P/3 is completely absent (Rosières 1, Crégols) or present in small proportions [Mémerlin-Muséum (1/13); Escamps A (1/3), B (1/3), C (1/15)], and also, the presence of a third ectoloph branch on M3/ (not found at Mémerlin, where P/3 is absent) or coexistence of different morphotypes (based on the development of this third branch) at Clapassou.
Palaeophyllophora oltina DELFORTRIE, 1873 (Plate 19)
Palaeophyllophora oltina (Delfortrie 1872) from Ste-Néboule: a SNB_PoA.1.4, left C/1, labial view (left) and lingual view (right). b SNB-80, fragment of left hemimandible with alveoli for I/1–2, C/1 and P/2–3, and with P/4 and M/1. c SNB-79, fragment of left hemimandible with M/2–3. d SNB-85, right C1/, labial view (left) and lingual view (right). e SNB_PoA.1.5, right P4/. f SNB_PoA.2.6, left M1/. g SNB_PoA.2.4, right M2/. h SNB-88, right M3/
Synonymy: 1873: Vespertilio oltinus in Delfortrie, p. 511–518, fig. 1–10
1876: Vespertilio oltinus in Gervais, p. 57
1896: Vespertilio oltinus in Roger, p. 29
1898: Vespertilio oltinus in Trouessart, p. 128
1904: Vespertilio oltinus in Trouessart, p. 91
1917: Palaeophyllophora sanctae-neboulae in Revilliod, fig. 1–3 Pl. 1, p. 43.
1987: Palaeophyllophora cf. oltina in Remy et al., tabl. 2a, p. 180
Previous references: 1978: Palaeophyllophora oltina in Sigé, p.258–261, Pl. 1 fig. 5, Pl. 2 fig. 1–6
2006: Palaeophyllophora oltina in Sigé & Crochet, p. 197
Amended diagnosis (no diagnosis has been provided until the present work, undertaken on a relatively substantial body of material): the largest Palaeophyllophora species described to date; P/3 vestigial but present; upper molars with mesostyle set back and protruding labial cingulum.
Type: As lectotype, Sigé (1978) proposes the unnumbered cranium featured by Delfortrie (1873), fig. 1, p. 712), from the UM2 collections.
Type-locality: Ste-Néboule de Béduer, Old Quercy Collections (exact locality unknown, indeterminate age), from the collections of the Muséum d’Histoire Naturelle de Genève.
Reference population: Ste-Néboule (MP 18), Lot, Phosphorites du Quercy, France
Material and measurements: see Appendix 4.
Remarks: to date, this species is only present at the Ste-Néboule locality (sampled/excavated by researchers in the first years of the 1970s). The quantity of collected material has increased since the publication of the Ste-Néboule monograph (Gèze et al. 1978), and it is now possible to describe this taxon in more detail and thus to consider this population as the reference one for P. oltina.
Description: Mandible: horizontal ramus approximately one and a half times the depth of molar crown; extended masseteric fossa; quasi-vertical ascending ramus where the base, elevated compared to the horizontal ramus, is slightly concave ventrally, the condyle elongated; wide mental fossa (Fig. 24a). Two lower incisors indicated by the number of alveoli, potentially of large size.
C/1: large, surrounded by slightly oblique, thick cingulid (rising anteriorly), becoming even more significant lingually; posterolingually directed apex; posteriorly concave in labial view, can be marked by wear; anterior surface convex and smooth.
P/2–3 indicated by the alveoli anterior to P/4; both uniradiculate; P/2 slightly smaller than P/4.
P/3 vestigial and closer to the labial side of the jaw.
P/4 wide with pointed occlusal contour anteriorly, transverse posteriorly; encircling cingulid, slightly sinuous on the labial and lingual sides, and widening anterolingually and more so posteriorly; dominant protoconid; incipient metaconid.
M/1–2 large, with trigonid wider than talonid; small basin, necromantodont in structure with a hypoconulid of almost equivalent size to entoconid; trigonid of M/1 transversely compressed compared to that of M/2, but generally wide open on all lower molars.
M/3: trigonid less reduced than on M/2; talonid extremely narrow and short with very small entoconid, hypoconid and hypoconulid.
C1/ large; composed of a lamella flanked at the base by a ridge to the front and lingual areas, where it also gets wider; convex labial aspect; almost flat lingual face.
P4/ with rectangular occlusal shape; powerful paracone with less extended postparacrista; heel developed lingual and posterior to paracone; surrounding cingulum.
M1/ large; short internal ectoloph branches setting the mesostyle well back from the labial edge; protofossa reduced and shallow, slightly open posteriorly with postprotocrista net meeting the metacone base; preprotocrista connected to the precingulum, itself meeting the strongly developed straight parastyle; heel extended posteriorly, with thick cingulum connected to the postcingulum; distinct labial cingulum of variable length.
M2/: identical morphology to M1/; protofossa closed by the connection between the postprotocrista and base of metacone; anterior edge of the tooth as wide as posterior edge.
M3/ simple; only two ectoloph branches; dominant paracone, parastyle and protocone; cingulum surrounding tooth, stopping posteriorly before reaching the parastyle.
Comparison: this is the largest known species of the genus Palaeophyllophora. There are also morphological differences from P. rosierensis nov. sp., notably the shallower horizontal ramus, the ascending ramus being lower, the mental fossamore reduced, the masseteric fossa less extended and markedly deeper, and also the wider upper molars bearing a mesostyle set back from the labial edge, a more imposing P/4, lower molars with more reduced talonids, better developed hypoconulids, and M/3 with shorter talonids. With regard to other species, body size provides an obvious distinction despite the morphology being similar to that of P. quercyi and P. parva nov. sp.
Palaeophyllophora quercyi REVILLIOD, 1917 (Plate 20a–e)
Palaeophyllophora quercyi Revilliod 1917 from Salème, Bouyssou 2 and Ginouillac: a SA_PqF.3.10, left P/4 with marked occlusal contour. b BOU2_PqA.1.1, fragment of left hemimandible with alveoli for I/1–2, C/1, and P/2–3, and with P/4 and M/1–2–3 with a more open trigonid and more reduced talonid. c GIN_PqA.1.3, right M1/ with a more labially positioned mesostyle. d SA_PqF.2.8, right M3/, shorter and with a posteriorly projecting mesostyle. e BOU2_PqA.1.7, left M3/ with more reduced protofossa and posteriorly projecting mesostyle. Palaeophyllophora nova nov. sp. From Aubrelong 1: f ABL1_PnC.3.2, left C/1, labial view (left) and lingual view (right). g ABL1_PnB.2.10, anterior part of a left hemimandible with I/1–2, C/1 and P/2–3–4. h ABL1_PnA.2.2, holotype, left hemimandible with alveoli for C/1 and P/2, and with P/4 and M/1–2–3. i ABL1_PnC.2.4, left C1/, labial view (left) and lingual view (right). j ABL1_PnA.2.8, fragment of maxillary with alveolus for C1/, and with P2–4/ and M1–2–3/
Synonymy: 1979: Palaeophyllophora quercyi in Sigé et al., p. 47, 92
1987: Palaeophyllophora quercyi and cf. in Remy et al., tabl. 1a, p. 177
1987: Palaeophyllophora cf. oltina in Remy et al., tabl. 1a and 2a, p. 177 and 180
Previous references: 1967: Palaeophyllophora quercyi in Miguet, p. 109–110
1978: Palaeophyllophora quercyi in Sigé, p.255–258, Pl. 1 fig. 3–5
1981: Palaeophyllophora quercyi in Crochet et al., tabl. 2-2
1988: Palaeophyllophora quercyi in Sigé, p. 89, text-fig. 24
2006a: Palaeophyllophora quercyi in Maitre et al., p. 116–118, fig. 5a
2006: Palaeophyllophora quercyi in Sigé & Crochet, p. 198
Original diagnosis: Revilliod specifies that the description of this species is that of the genus.
Type: Revilliod (1917) failed to designate a type for this species, and Sigé (1978) proposed the most complete specimen as type: the lectotype, namely Q.P. 884, is the left maxillary, figured by Revilliod (1917), from the collections of the Naturhistorisches Museum Basel.
Type-locality: Old Quercy Collections (unknown locality, indeterminate age).
Reference population: Ste-Néboule (MP 18), Lot, Phosphorites du Quercy, France
Other localities: Le Bretou (MP 16), Lébratières 1, Ginouillac, Salème, St-Antonin-Noble-Val, Aubrelong 2, Clapassou (MP 17a), Perrière, Malpérié (MP 17b), Bouyssou 2, Théron, Monteils (MP 18), Rosières 1–3, Palembert, Célarié ocre, Célarié standard (MP 19).
Material and measurements: see Appendix 4.
Description: Mandible: large ascending ramus, relatively tall and abruptly becoming subvertical posterior to M/3; concave ventral margin, slightly elevated compared to that of the horizontal ramus; deep masseteric fossa; condyle thin but long; angular process wide and directed ventrally; dentary slightly taller than the height of a slightly worn molar crown (Fig. 24b).
The dental characteristics given by Sigé (1978) complete the initial description by Revilliod (1917). Added here are remarks concerning the variability found within the material from the studied localities.
Comparison: the only tooth found at Le Bretou, a right C1/ (Sigé 1988, p. 89, text-fig. 24) is highly polished, but the remains of a thick cingulum can be seen under low-angled light on the labial aspect and more clearly on the lingual aspect.
The lower molars from Lébratières 1 and Bouyssou 2 have a slightly more reduced talonid than those of the molars from Ste-Néboule. In the second locality, they have a paraconid that is more extended anteriorly, and M3/ has a very reduced protofossa with a mesostyle posteriorly inclined.
At Salème, P/4 has a less square occlusal shape, with the anterior part of the tooth extending anteriorly, and having a larger subhorizontal postprotoconid surface. M3/ has a mesostyle that leans posteriorly slightly, and is shorter than those from Ste-Néboule.
The mesostyle of the upper molars from Ginouillac is closer to the labial edge, the internal ectoloph branches being longer.
The fauna from Rosières 1 comprises three species of the genus Palaeophyllophora with slightly overlapping size ranges. The rather small average dimensions of P. quercyi in this locality point to the difficulty to assign specimens of intermediate size, especially as their morphologies are so similar.
Smaller than P. nova nov. sp., the ascending ramus of P. quercyi is longer and the angular process wider. The lower molars have a less open trigonid and a larger talonid, with a more lingual hypoconulid (less necromantodont). P. quercyi also differs in its bifurcated P2/, P4/ with a shorter heel, upper molars with a more extended protofossa, sometimes open posteriorly, and clearly longer premetacrista and postparacrista. The heel is still more reduced. M3/ is not as short, and its protocone is stronger.
The morphology of P. quercyi is very close to that of P. parva nov. sp., and this species differs mainly in its larger size.
Finally, P. quercyi differs from P. rosierensis nov. sp. in its smaller size, lower molars with a more reduced talonid and upper molars with a heel that is less posteriorly extended.
Palaeophyllophora nova nov. sp. (Plate 20f–j)
Synonymy: 1970: Palaeophyllophora sanctae-neboulae in Lange, p. 152
1973: Palaeophyllophora quercyi in de Bonis et al., tabl. 2a
1979: Palaeophyllophora oltina in Sigé et al., p. 47, 87
1987: Palaeophyllophora quercyi in Remy et al., tabl. 3a p. 183
2006: Palaeophyllophora quercyi in Sigé & Crochet, p. 198
Diagnosis: Palaeophyllophora of average size, with clear reduction of talonid and protofossa, and a mesostyle well removed from the labial edge, with shorter internal ectoloph branches than other species of the genus.
Derivatio nominis: from the Latin novus: new, due to the appearance of this species around the time of the Grande Coupure, therefore later than other species of this genus, with markedly different morphological characters.
Holotype: MGT_PnA.2.2, left hemimandible bearing P/4–M/3, Pl. 20 Fig. h, from the UM2 collections.
Type-locality: Mas de Got (MP 22) Lot, Phosphorites du Quercy, France.
Other localities: Cloup d’Aural 1, Lpbratiqres 2 (post MP 20), Ravet-Lupo Aubrelong 1 (MP 21), La Couaille, La Plante 2, Jammes, Baraval, Cavalé, Les Bories, Lébratières 12-13-15 (MP 22), Gardiol 3 (MP 23).
Material and measurements: see Appendix 4.
Description: Mandible: dentary of depth similar to that of molar crown; wide mental fossa; ascending ramus rising abruptly posterior to M/3, to reach the height of roughly three molar crowns; base of the ramus concave ventrally and slightly elevated compared to the horizontal ramus; deep masseteric fossa; long, thin condyle; angular process directed ventrally (Fig. 24c).
I/1–2 uniradiculate, with three lobes.
C/1 typical of the genus with surrounding cingulid, wider anterolingually and posteriorly.
P/2 with oval occlusal shape, ending in slight point at anterior and posterior extremities.
P/3 vestigial, mostly present (only 2 specimens out of 35 are lacking P/3); deviated on the labial edge; with a single root very similar to that of P/2, and sometimes even joined alveoli. P/4 typical of the genus with a cingulid surrounding the tooth, slightly sinuous and labially oblique (lower posteriorly); comprising a convex anterolabial surface and flat anterolingual and posterior faces, with a developed subhorizontal surface at their base.
Necromantodont lower molars with quite open trigonid, and very reduced talonid (Fig. 25).
C1/ bulky; thick lamella surround by cingulum, thickening anteriorly and lingually, where it is also wider; convex labial aspect; flat lingual face.
P2/ vestigial, with oval occlusal contour and generally fused roots.
P4/ composed of a massive paracone from where begins a postparacrista, a slight parastyle and a well-developed posterior heel, sometimes with two lobes; slight cingulum surrounding the tooth.
M1–2/ has very reduced, closed protofossa with very short internal ectoloph branches; cingulum surrounding tooth except at the anterolingual corner; well-developed heel, posteriorly extended and bearing a thick cingulum connected to the postcingulum (Fig. 25b).
M3/ much narrower than M1–2/, with two ectoloph branches, an almost nonexistent protofossa, a parastyle and a mesostyle inclined posteriorly; presence of pre- and postcingulum, fading near the mesostyle and parastyle.
Comparison: the Ravet deposit produced only a single M/3 but the reduction of the talonid, still more pronounced than that of P. quercyi, suggests it belongs to this taxon.
Slightly larger than that of P. quercyi, the ascending ramus of the hemimandible of P. nova nov. sp. is shorter, and the angular process narrower. Other differences include P2/ with fused roots, P4/ with longer heel, upper molars with reduced protofossa, more closed posteriorly (except for specimens from Gardiol 3), and with shorter internal ectoloph branches, heel better developed and posteriorly extended. M3/ clearly smaller with a very reduced protocone and protofossa. P/4 is slightly more extended anteriorly. The lower molars have a trigonid that is more open, and a talonid more reduced, with a median hypoconulid.
Palaeophyllophora parva nov. sp. (Plate 21)
Palaeophyllophora parva nov. sp. from Mas de Labat 1 and Baraval: a MAB1_PpE.1.4, right C/1, labial view (left) and lingual view (right). b MAB1_PpD.2.5, P/2. c MAB1_PpB.4.3, fragment of right hemimandible with alveoli for C/1 and P/2, and with P/4 and M/1–2. d MAB1_PpD.1.9, fragment of left hemimandible with one alveolus for M/2, and with M/3. e BAR_PpA.2.1, right M/3 with more open trigonid and more reduced talonid. f MAB1_PpF.1.4, left C1/, labial view (left) and lingual view (right). g MAB1_PpA.1.6, holotype, right maxillary of the type skull with alveolus for P2/, and with broken C1/, P4/ and M1–2–3/
Synonymy: 1973: Palaeophyllophora sp. in de Bonis et al., tabl. 2a
1981: Palaeophyllophora cf. quercyi in Crochet et al., tabl. 2-2
1987: Palaeophyllophora cf. quercyi in Remy et al., tabl. 1a and 2a p. 177 and 180
1995: Palaeophyllophora quercyi in Legendre et al., p. 64–65
1998: Palaeophyllophora quercyi in Sigé et al., p. 87
2000: Palaeophyllophora quercyi in Astruc et al., table 1 p. 278
2006a: Palaeophyllophora quercyi (pro parte) in Maitre et al., p. 117, fig. 5a
Diagnosis: morphologically similar species to P. quercyi, but of smaller size.
Derivatio nominis: from the Latin parvus: small, as this is the smallest known species of this genus.
Holotype: MAB1 PpA.1.6, cranium and left and right maxillae both bearing C1/–M3/ (Plate 21g), from the UM2 collections.
Type-locality: Mas de Labat 1 (MP 18), Lot, Phosphorites du Quercy, France.
Other localities: Les Pradigues (MP 17a), Malpérié, Pépénut (MP 17b), Théron, Mémerlin-Muséum, Crégols, Sindou D (MP 18), Guirolle rouge, Rosières 1–2, Escamps, Guirolle blanc (MP 19), Tabarly (MP 20), Coyrou 1-2 (MP 20/21), Mas de Labat 2 (MP 21), Baraval (MP 22).
Material and measurements: see Appendix 4.
Description: Mandible: subvertical ascending ramus, rising abruptly posterior to M/3 and with a ventral margin slightly higher than the horizontal ramus; deep masseteric fossa; thin and long condyle; angular process facing ventrally, widening at the extremity (Fig. 24d). I/1–2: only indicated by the alveoli anterior to C/1.
C/1 surrounding cingulid, widening posteriorly with a projection at the antero- and posterolingual corners; anterior aspect convex, posterior face flat; posterolingually facing apex.
P/2 uniradiculate; oval occlusal shape; anteriorly and posteriorly pinched; surrounded by thick, wide cingulid.
P/3 absent.
P/4 variable occlusal shape, relatively extended anteriorly; surrounded by thick cingulid except at the level of the metaconid, beginning on the lingual edge and widening anterolingually and posteriorly at the protoconid.
M/1–2 open trigonid with talonid slightly reduced in comparison; entoconid slightly higher than hypoconulid, separated by a sinus.
M/3 smaller than M/2; more reduced talonid, composed of an entoconid, a hypoconid and a hypoconulid, barely cuspidate.
C1/ massive; typical of the genus with a thick and wide lingual cingulum, fading labially until it completely disappears; flat lingual aspect; convex labial aspect.
P2/ biradiculate; vestigial; one lingual root and one labial root.
P4/ typical of the genus in having a dominant paracone with a postparacrista, sometimes a comparatively cuspidate parastyle, a protocone usually in good relief, a surrounding cingulum, and a heel developed posterior to the paracone; posterior edge sometimes anteroposteriorly indented.
M1–2/ distinctly rectangular; somewhat developed heel, extended posteriorly, with a thick cingulum; protofossa generally open on M1/ and closed on M2/; mesostyle set in from labial edge, as the internal ectoloph branches are short.
M3/ reduced to 2 ectoloph branches; cuspidate protocone and parastyle, connected by a cingulum, unbroken to the mesostyle.
Comparison: the occurence of P/3 varies greatly. In many localities specimens have none, or very few (Mémerlin-Muséum, Crégols, Mas de Labat 1, Rosières 2, Rosières 1, Mas de Labat 2). This vestigial tooth seems to disappear progressively over time, from reference level MP 18 onwards.
At Perrière, the mesostyle of the upper molars is relatively close to the labial edge. At Baraval, the only available M/3 displays a less transverse trigonid and a clearly shorter talonid adpressed against the trigonid, with a hypoconid that is closer to the protoconid. Both specimens found at Théron are likely to belong to P. parva based on their morphology, despite the fact that they are unusually small.
The Rosières 1 bat fauna has three species of the genus Palaeophyllophora with slightly overlapping size ranges. The relatively significant average size of P. parva nov. sp. in this locality is proof of the difficulties in the attribution of species of intermediate size, more so as the morphology is similar between these three species. In comparison to P. nova nov. sp., this species is not only smaller, but also has a shorter ascending ramus and a more ventrally directed angular process. Morphologically very similar to P. quercyi, it differs from P. nova nov. sp. in the following characters: less reduced talonid and more lingual hypoconulid on lower molars; larger protofossa and longer internal ectoloph branches on upper molars.
Palaeophyllophora rosierensis nov. sp. (Plate 22a–e)
Palaeophyllophora rosierensis nov. sp. from Rosières 2: a ROS2_PrC.4.3, left C/1, labial view (left) and lingual view (right). b ROS2PrB.4.14, right P/2. c ROS2_PrB.1.3, fragment of right hemimandible with alveoli for C/1 and P/2, and with P/4 and M/1–2–3. d ROS2_PrC.1.5, left C1/, labial view (left) and lingual view (right). e ROS2_PrB.2.1, holotype, fragment of right maxillary with alveoli for C1/ and P2/, and with P4/ and M1–2–3/. f Palaeophyllophora sp. B from Cuzal: CUZ_PspBA.1.3, right P/4
Synonymy: 1973: Paleophyllophora aff. oltina in de Bonis et al., tabl. 2a
1981: Paleophyllophora aff. oltina in Crochet et al., tabl. 2-2
1987: Palaeophyllophora sp. in Remy et al., tabl. 1a, p. 177
1987: Palaeophyllophora oltina and cf. in Remy et al., tabl. 2a and 3a, p. 180 and 183
2000: Palaeophyllophora sp. in Astruc et al., table 1, p. 278
2006a: Palaeophyllophora oltina and P. sp. in Maitre et al., p. 116 and 118, fig. 5a
Diagnosis: species of intermediate size between P. oltina and P. quercyi.
Derivatio nominis: from the names of two localities where this species is well represented: Rosières 1 and 2, of which the latter is the type-locality.
Holotype: ROS 2 PrB.2.1, right maxilla showing the alveoli of C1/ and P2/, and bearing P4/–M3/, (Plate 22e), from the UM2 collections.
Type-locality: Rosières 2 (MP 19), Lot, Phosphorites du Quercy, France.
Other localities: Bouziès (MP 17a), Mas de Bonhomme, Bouyssou 3 (MP 17b), Théron, Liauzu, Mémerlin-Muséum, Crégols, Mas de Labat 1, Sindou D (MP 18), Guirolle rouge, Rosières 1, Coânac 1, Escamps, Nougayrac, Lostange, Guirolle blanc (MP 19), Pécarel, Tabarly (MP 20), Coyrou 1-2, (MP 20/21), Mas de Labat 2, Garrhan (MP 21), Lébratières 12 (MP 22).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus depth of roughly one molar crown; robust ascending ramus, subvertical; deep masseteric fossa; thin and long condyle; angular process facing ventrally (Fig. 24e).
We find again the dental morphology typical of the genus: Presence of two incisors indicated by the alveoli anterior to C/1. C/1: surrounded by cingulid thickening lingually and slightly sinuous labially; apex facing slightly posteriorly.
P/3 absent or vestigial and uniradiculate (none in specimens from Rosières 2 or Escamps, 2/4 at Bouziès).
P/4 square occlusal contour; flanked by cingulid widening anterolingually and posteriorly; dominant protoconid; incipient metaconid. Lower molars: reduced talonid, with entoconid and hypoconulid (somewhat median) of similar volume, separated by a sinusid; M/3 talonid very reduced with discrete cusps.
C1/ large; cingulum present labially, strongly developed on the lingual edge and slightly going up the lamella anteriorly.
P2/ bicuspidate; labial and lingual roots; present as stylet.
P4/ bulky; strong parastyle; long heel, marked by a small basin; flanked by a cingulum.
M1–2/ comparatively square occlusal outline; protofossa generally open on M1/ and closed on M2/ variable distance of mesostyle from labial edge, with internal ectoloph crests being shorter compared to external branches.
M3/ reduced, with two ectoloph crests; dominant paracone; relatively reduced protocone; surrounding cingulum, disappearing at the parastyle and mesostyle; very reduced protofossa, almost without basin.
Comparison: intraspecific variation typical of that observed for other species: the occlusal shape of P/4 more or less extended anteriorly; somewhat median position of hypoconulid on the lower molars; development of the heel on upper molars; development of the parastyle and relatively significant anteroposterior compression on P/4; accentuated indentation of upper molar labial edge; the length of M3/.
The specimens from Rosières 1 are of intermediate size between those of Escamps, being larger, and those of Rosières 2, being smaller. Their size is clearly smaller than that of P. oltina, which can also be distinguished by the longer horizontal ramus, the taller ascending ramus, the wider mental fossa, a larger, shallower masseteric fossa, a more reduced talonid on the lower molars, on the upper molars a mesostyle that is more labially inset and a heel where the cingulum is generally thicker. The size of P. rosierensis nov. sp. is clearly greater than that of P. quercyi from Ste-Néboule, which also has more reduced talonids, and heels that are less posteriorly extended.
? Palaeophyllophora sp. A
Previous references: 1997: ? Palaeophyllophora sp. in Sigé, p. 745, fig. 9, tabl. 1.
Locality: St-Maximin (MP 13), Phosphorites du Gard, France.
Material and measurements: see Appendix 4.
Remarks: No additional material was found in this study, and hence two specimens, a P4/ and a C1/, are assigned to the genus Palaeophyllophora with only the details provided by Sigé (1997).
Palaeophyllophora sp. B (Plate 22f)
Locality: Cuzal (MP 13), Lot, Phosphorites du Quercy, France.
Material and measurements: see Appendix 4.
Description: large P/4; trapezoidal occlusal contour, narrower anteriorly; surrounded by a cingulid, thick and labially sinuous, wide and flanked posteriorly by a deep sinusid, ending with a small swelling at the posterolingual corner; distinct preprotocristid, also ending in a swelling at the lingual edge.
Comparison: the morphology is exactly that of the P/4 of P. oltina or P. quercyi, recorded several reference levels later (ca. 4 million years). It is slightly smaller than that of P. oltina from Ste-Néboule, and distinctly larger than P. quercyi from the same locality, and potentially also from St-Maximin.
General remarks on the genus Palaeophyllophora:
The existence of these three teeth, clearly referable to the genus Paleophyllophora, documents the presence of this form as early as the middle Eocene (MP 13), whereas a single upper canine was found at Le Bretou (MP 16), and the first effective population indicators are only recorded from reference level MP 17a. Thus, this genus seems to be the first member of family Hipposideridae to appear within Eocene chiropteran faunas. It already exhibited a certain amount of diversity including potentially large forms, as indicated by the specimen from Cuzal.
Genus Vaylatsia SIGÉ, 1990
Dental formula: I 1?/2, C 1/1, P 2/3, M 3/3.
Original diagnosis: Hipposideridae with dental formula I 1?/2, C 1/1, P 2/3, M 3/3, retaining a relatively significant vestigial P2/ and a labial, vestigial P/3; C/1 where the vertical axis of the cusp is tilted on the axis of the root (in lateral view), with clear, sinuous labial cingulum and mesially ascendant lingual cingulum; P4/ where the lingual cingulum extends the lingual crest of the paracone; upper molars with labial mesostyle, where pre- and postprotocristae are connected to the para- and metacingulum, respectively; lower molars with relatively long talonid, wide and tall; M3/3 less reduced; distal humerus with spherical condyle.
Type-species: Vaylatsia garouillasensis Sigé, 1990
Other species described: ?V. lemanensis, V. garouillasensis, V. pumilio, V. prisca, V. pelissiei nov. sp., V. astruci nov. sp., V. valettei nov. sp., V. frequens nov. sp., V. cregolensis nov. sp.
Distribution: from the basal upper Eocene (MP 16) to the basal upper Oligocene (MP 25) in western Europe (France).
Vaylatsia prisca (REVILLIOD, 1920)
Synonymy: 1920: Rhinolophus priscus in Revilliod, fig. 14–16, p. 64–66.
Remarks: at the creation of Rhinolophus (presently Vaylatsia) priscus, Revilliod (1922) grouped under the same name four Quercy specimens of indeterminate origin, and one mandible from Mormont (Eclépens), without nominating a holotype. All of these specimens automatically constitute the syntypes of V. prisca (Zoological Nomenclature Code p. 212 art. 73.2). For the lectotype of the species Rh. priscus, Hooker and Weidmann (2000) retained the mandible from Mormont, Mt. 992. However, the material from the collections of the Naturhistorisches Museum Basel is heterogenous: Mt 992 is much larger than the recently obtained specimens from the Quercy, which are homogenous, and larger than the largest Quercy Vaylatsia species. Furthermore, the single reference to “Eclépens” made by Revilliod is not sufficient to pinpoint the location or its age, given that the reference corresponds to several deposits spreading from the end of the Bartonian to the late Priabonian. However, the choice made by Hooker and Weidmann is valid, and this mandible thus becomes the lectotype of the species V. prisca, the Swiss locality Eclépens becoming the type-locality. The mandibles Q.P. 747 and Q.H. maxillaries 88, 228 and 200, of homogeneous size and morphology, but different from that of V. prisca, are attributed to a new species described below, V. pelissiei nov. sp.
Vaylatsia pelissiei nov. sp. (Plate 23a–d)
Synonymy: 1979: Rhinolophus priscus in Sigé et al., p. 48
1973: Rhinolophus cf. priscus in de Bonis et al., tabl. 2a
1987: Rhinolophus cf. priscus in Remy et al., tabl. 3a p. 183
1998: Vaylatsia cf. prisca in Sigé et al., p. 87
Previous reference: 2006: Vaylatsia prisca in Sigé & Crochet, p. 198
Diagnosis: Vaylatsia of intermediate size between V. astruci and V. frequens; C1/ with pronounced indentation of labial edge; M3/3 less reduced, relatively long upper molars.
Derivatio nominis: in honour of Thierry Pélissié who, through his speleological expertise and consideration for fossils, has made a long, important and very significant contribution to the discovery and collection of many and various fossiliferous paleokarstic filling sediments in the Quercy.
Type: Q.H. 200, left and right maxillae still fused and displaying the alveoli of C1/ and the left P2/, and bearing the left P4/–M1–2–3/ and the right P2–4/–M1–2–3/.
Reference population: Mas de Got (MP 21), Lot, Phosphorites du Quercy, France.
Other localities: La Plante 2, Baraval, Cavalé, La Nauze 1 (MP 22), Gardiol 3 (MP 23).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus depth equivalent to roughly one molar crown; mental fossa anterior to P/2; ascending ramus height equivalent to barely twice the height of a molar crown, ascending slowly posterior to M/3, then quickly becoming vertical; small masseteric fossa; the base of the ascending ramus elevated but horizontal; angular process much extended ventrally, and laterally (Fig. 26a).
Lower incisors not observed, only indicated by the alveoli anterior to C/1.
C/1 typical of the genus, surrounded by a cingulid widening posteriorly.
P/2 uniradiculate; almost as long as P/4; oval occlusal contour, slightly pinched anteriorly and posteriorly; surrounded by cingulid.
P/3 vestigial, but more or less reduced in size.
P/4 composed of a protoconid, a rudimentary metaconid and a developed cingulid, distinctly wider around the perimeter, except at base of metaconid.
M/1–2 nyctalodont; trigonid widely open, mainly on M/1; talonid slightly offset labially compared to the trigonid; concave pre-entocristid; hypoconulid slightly projecting posteriorly.
M/3 barely smaller than M/2; trigonid less open; slightly reduced talonid; cuspidate hypoconulid. C1/ with very indented labial edge; lingual cingulum very thick and wide; small cusp at the base of the posterior edge.
P2/ biradiculate; oval occlusal shape, slightly pinched anteriorly and posteriorly.
P4/ showing a paracone with prominent postparacrista; developed parastyle, forming a projection on the anterior edge of the tooth; heel largely developed posteriorly, and with a basin flanked by a cingulum; slightly cuspidate protocone.
M1/ strong heel, somewhat lingually extended and with pronounced cingulum; M2/ smaller, with a heel more contained than on M1/; internal ectoloph branches as long as external branches, so mesostyle projects from labial edge; pre- and postprotocristae respectively connected to the pre- and postcingulum.
M3/ not very reduced with three ectoloph branches and sometimes the beginning of a fourth; labial edge not very oblique due to postparacrista and premetacrista being almost as long as the preparacrista; well-developed parastyle; extended protofossa; protocone connected to pre- and postcingulum.
Comparison: V. pelissiei nov. sp. from Gardiol 3 seems to be quite small in comparison to the measurements generally observed. In comparison to V. astruci nov. sp., V. pelissiei nov. sp. is larger, P/4 with a square occlusal shape; M/3 longer with a cuspidate hypoconulid; M3/ equally as long with a clearly longer third ectoloph branch; C1/ with a much more indented labial edge; more transversely developed P4/ and upper molars.
V. pelissiei nov. sp. differs from V. frequens nov. sp. in its smaller size; P/4 with less developed cingulum; trapezoidal lower molars; and P2/ and M3/ being respectively less reduced and longer.
Vaylatsia garouillasensis SIGÉ, 1990
Previous references: 1995: Vaylatsia garouillasensis in Sigé, p. 89–95, text-fig. 13–18
2006: Vaylatsia garouillasensis in Sigé & Crochet, p. 194
Original diagnosis: that of the genus; average dimensions.
Type: GAR 2439, fragment of left hemimandible with the alveoli of C/1–P/4, and bearing well preserved, hardly worn M/1–M/3.
Type-locality: Le Garouillas (MP 25), Lot, Phosphorites du Quercy.
Other localities: Belgarric, Rigal-Jouet, Phalip, La Garrigue, L’Escoufle, Ppcofi (MP 25).
Measurements: see Appendix 4.
Description/Comparison: not given here, given that no new specimens in this study were assigned to this taxon. Thus the remarks by Sigé (1995) remain complete and sufficient.
Vaylatsia pumilio REVILLIOD, 1920
Synonymy: 1920: Rhinolophus pumilio in Revilliod, fig. 17 p. 66–67
1967: Rhinolophus pumilio in Miguet, p. 110–111
1995: Vaylatsia mvli in Sigé, p. 95–100, text-fig. 19–23
2006: Vaylatsia mvli in Sigé & Crochet, p. 196
Original diagnosis (Revilliod having failed to provide a diagnosis, that of V. mvli, a junior synonym of V. pmilio, see below, is given here): clearly smaller than V. garouillasensis.
Amended diagnosis: species of intermediate size between V. cregolensis and V. astruci; M2/ less transversely developed/narrower; very slight heel given relief by a wide cingulum connected to the postcingulum.
Holotype (monotype): Q.P. 999, fragment of right mandible with the alveoli of I/1–2, C/1, P/2–3 and bearing P/4, M/1–2–3; Revilliod (1920) from the collections of the Naturhistorisches Museum Basel.
Type-locality: Old Quercy Collections (unknown locality, indeterminate age).
Reference population: Le Garouillas (MP 25), Lot, Phosphorites du Quercy, France.
Other localities: La Couaille (MP 20/21), Lébratières 15 (MP 22), Lébratières 14 (MP 24), Belgarric, Rigal-Jouet, La Plante 3, La Garrigue, L’Escoufle (MP 25)
Added material and measurements: see Appendix 4.
Remarks: this work has helped highlight the synonymy between Revilliod’s species, R. pumilio, and the species V. mvli created by Sigé (1990). Modern knowledge shows that this type of dentition is associated with a humerus having a rounded condyle, with a strong functional implications (Sigé 1966). Thus, the attribution to the genus Rhinolophus is contradicted, whereas Sigé’s proposal of a distinct genus, Vaylatsia, is necessary. The description of this taxon will not be repeated here, since it has been fully provided by Sigé (1990, 1995).
Comparison: the specimens newly attributed to this species are morphologically identical, in the comparable dental categories (M/1, C1/ and M2/). This species differs from V. cregolensis nov. sp. in its larger size and proportionally less transversely developed M2/, with practically no heel.
Vaylatsia astruci nov. sp. (Plate 23e–j)
Vaylatsia pelissiei nov. sp. from Mas de Got: a MGT_VapA.2.9, right C/1, labial view (left) and lingual view (right). b MGT_VapA.2.17, right hemimandible with alveoli for I/1–2, and with P/2–3–4 and M/1–2–3. c MGT_VapB.2.12, right C1/, labial view (left) and lingual view (right). d MGT_VapA.1.3, fragment of left maxillary with C1/, P2–4/ and M1–2–3/. Vaylatsia astruci nov. sp. from Bretou and Aubrelong 2: e BRE_2-790, holotype, fragment of left with alveoli for I/1–2, C/1 and P/2–3, and with P/4 and M/1–2. f BRE_1-772, fragment of right hemimandible with M/1–2–3. g BRE_2-836, left C1/, labial view (left) and lingual view (right). h BRE_1-775, fragment of right maxillary droit with alveolus for C1/, and with P2–4/ and M1–2. i ABL2_VaaA.2.6, right M1/ with posteriorly stretched heel. j BRE_1-787, left M3/
Synonymy: 1974: Rhinolophus sp. in Hartenberger et al., p. 193
1979: Rhinolophus antiquus in Sigé et al., p. 48, 87
1981: Rhinolophus sp. 1 in Crochet et al., tabl. 2-2
1987: Rhinolophus sp. in Remy et al., tabl. 1a et 2a p. 177 et 180
1988: Rhinolophoïdea sp. indet. in Sigé, p. 93–97, text-fig. 32–35
2006a: Vaylatsia sp. C in Maitre et al., p. 117, fig. 5a
Diagnosis: pronounced reduction of M3/3; strong variability of the ectoloph on M3/; smaller than V. pelissiei, but clearly larger than V. cregolensis.
Derivatio nominis: in honour of Dr. Jean Guy Astruc, who, as well as his contributions to the understanding of the formation of karstic network, and the development of fillings in the field of geology, pointed out to palaeontologists many fossil deposits (including St-Antonin-Noble-Val, St-Lizier, Pépénut, Souhic, etc.).
Holotype: BRE_2-790, fragment of left hemimandible with the alveoli of I/1–2, C/1, P/2, P/3 and bearing P/4–M/2 (Plate 23e), from the UM2 collections.
Type-locality: Le Bretou (MP 16), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: St-Lizier, Lavergne (MP 16), Lébratières 1, La Bouffie, Les Clapiès, Aubrelong 2, Trifon, Les Pradigues (MP 17a), Rosières 5, Pépénut, Sorcières (MP 17b), Rosières 2, Célarié standard (MP 19).
Measurements: see Appendix 4.
Description: Two lower incisors, indicated by alveoli anterior to C/1.
P/2 and P/3 not known, but indicated by the alveoli anterior to P/4.
P/3 vestigial, based on the diameter of the alveolus; leaning towards the labial edge of the jaw.
P/4 composed of a protoconid and rudimentary metaconid; surrounded by a cingulid, except on lingual edge, widening at the anterolingual, anterolabial and posterior corners, where it is slightly raised.
M/1–2 nyctalodont; widely open trigonid; labially offset talonid; small but cuspidate hypoconulid; M/1 longer than M/2, with trigonid extending anteriorly.
M/3 trigonid slightly smaller than that of M/2; talonid much smaller compared to M/1–2, notably in width; hypoconulid rarely separate (Fig. 27).
C1/ typical of the genus; lamella strongly curved posteriorly; oblique labial edge, often sinuous, and raised anteriorly; lingual cingulum, widening anteriorly and ending in a projection on the labial edge.
P2/ monoradiculated; rectangular occlusal shape; one central cusp; one longitudinal crest; very reduced in comparison to P4/.
P4/ wide tooth; strongly anteroposteriorly pinched; well-developed parastyle, protruding anteriorly with respect to the anterior edge of the tooth; dominant paracone with prominent postparacrista; non cuspidate protocone; developed heel, somewhat wider posteriorly and flanked by a cingulum more labially connected to the postcingulum.
M1–2/ extended, closed protofossa; low protocone; parastyle and posterolabial corner of the tooth much extended labially; preprotocrista and postprotocrista connected to the parastyle and the posterolabial corner respectively; anterior edge of M1/ slightly narrower than posterior edge; developed heel, extending to varying degrees posteriorly, with cingulum of variable width, sometimes bearing a hypocone.
M3/ reduced tooth, transversely extended, with two large ectoloph branches, and posterior one distinctly shorter; extended protofossa closed by the pre- and postprotocristae, respectively connected to the pre- and postcingulum.
Comparison: the strong reduction of the M3/3 of V. astruci nov. sp. might suggest a stronger resemblance to Pseudorhinolophus. However, all the well-preserved hemimandibles attributed to this species have the alveolus of a vestigial P/3, leaning towards the labial edge and anterior to P/4. This character, always absent from the specimens of Pseudorhinolophus, is diagnostic of the genus Vaylatsia.
At Aubrelong 2, the heel of the M1/ is distinctly extended posteriorly and lingually. In general terms, all of the specimens are larger than those from Les Clapiès or La Bouffie, and smaller than those from Les Pradigues.
The M3/ trigon exhibits a strong variability: (1) the labial edge can be marked by an indentation between parastyle and mesostyle; (2) premetacrista can be developed to a varying degree as a projection on the posterior edge of the tooth; and thus (3) M3/ length varies. The smallest morphotype is found at Le Bretou or Lavergne localities, and the longest M3/ is found at St-Lizier. The specimens from Le Bretou are of intermediate size between those from St-Lizier (smaller) and those from Lavergne (larger) (Fig. 27b).
Finally, this species differs from Vaylatsia cregolensis nov. sp. by its larger P/3, shorter and wider P/4, M/3 with a more reduced talonid than generally seen in species of this genus, and a shorter M3/, with only three ectoloph branches. This species differs from V. pelissiei nov. sp. by being smaller, and having longer and narrower P/4, more reduced M3/3, C1/ with less indented labial edge, and more transversely developed P4/ and upper molars.
Vaylatsia valettei nov. sp. (Plate 24a–c)
Vaylatsia valettei nov. sp. from Tabarly and Escamps: a TAB_VavA.1.2, holotype, fragment of right hemimandible with alveoli for P/2–3, and with P/4 and M/1. b TAB_VvaA.1.1, right C1/, labial view (left) and lingual view (right). c ESCC_VvaA.1.1, right P4/. Vaylatsia frequens nov. sp. From Escamps and Coyrou 3: d ESC_VafF.2.5, right C/1, labial view (left) and lingual view (right). e COY3_VafA.2.13, right P/4. f ESCC_VafA.3.21, holotype, right hemimandible with alveoli for I/1–2 and C/1, and with P/2–4 and M/1–2–3. g ESCC_VafF.1.5, left C1/, labial view (left) and lingual view (right). h COY3_VafA.1.13, left P4/. i ESCA_VafA.1.6, fragment of right maxillary with C1/, P2–4/ and M1–2–3/. Vaylatsia cregolensis nov. sp. from Crégols, St-Lizier, Sindou D and La Bouffie: j CRE_VacA.1.1, holotype, fragment of right hemimandible with alveoli for I/2, C/1, P/2–3 and with P/4 and M/1–2. k SLI_VacA.1.3, left M/3. l SDD-1933, right M1/. m BFI_VacA.1.1, left M2/
Synonymy: 1973: Rhinolophus indet. 2 in de Bonis et al., tabl. 2a
1987: Rhinolophus sp. in Remy et al., tabl. 2a, p. 180
Diagnosis: species of Vaylatsia larger than V. garouillasensis, with lower dentition being wider, and C1/ without labial ridges.
Derivatio nominis: in honour of Cissou and Philippe Valette, always so welcoming to the palaeontologists in the Quercy and who, by their speleological expertise, greatly participated in the discovery and exploration of many fossiliferous fillings (e.g. those of Guirolle and Théron), and in their sampling.
Holotype: TAB VavA.1.2, fragment of right hemimandible with the alveoli of C/1, P/2, P/3, and bearing P/4–M/1, (Plate 24a), from the UM2 collections.
Type-locality: Tabarly (MP 20), Tarn-et-Garonne, Phosphorites du Quercy, France.
Other localities: Escamps (MP 19), Lot, Phosphorites du Quercy, France.
Material and measurements: see Appendix 4.
Description: P/2: uniradiculate; mental fossa at its base.
P/3 presence demonstrated by an alveolus with diameter as large as that ¾ of the depth of the ramus.
P/4: a single, well-worn tooth composed of a dominant protoconid and maybe a rudimentary metaconid; trapezoidal occlusal shape; surrounded by a sinuous cingulid, widening extensively anteriorly and posteriorly, where it is elevated lingually.
M/1 with talonid offset labially with respect to the trigonid; narrow, open trigonid; wide cingulid.
C1/ thick lamella, curving strongly posteriorly; coronal base sinuous labially, very indented at the anterolingual corner; thick, wide lingual cingulum, ending in a projection on the labial edge; labial aspect slightly convex, lingual face flat.
P4/ with a dominant paracone, a distinct parastyle projecting from the anterior edge of the tooth, incipient protocone, and well-developed heel; occlusal shape anteroposteriorly pinched; postcingulum better developed than precingulum.
Comparison: V. valettei nov. sp. is larger than V. garouillasensis, its lower teeth proportionally wider, and its C1/ do without labially marked cingulum.
In comparison to Rhinolophus lemanensis (Revilliod, 1920; fig. 18, p. 67–68), its size and morphology are relatively similar. However, the distinctly more recent assumed age of the type-locality of R. lemanensis (St-Gérand-le-Puy, Miocene, MN 2) makes it impossible to be fully confident in assigning this species to the genus Vaylatsia, given that the extant genus Rhinolophus could have been present during this time. The observation of R. lemanensis humerus would help to justify its generic classification. In the meantime, the previously described large specimens from Tabarly and Escamps cannot be assigned to this old taxon.
Vaylatsia frequens nov. sp. (Plate 24d–i)
Synonymy: 1973: Rhinolophus priscus in de Bonis et al., tabl. 2a
1978: Rhinolophus cf. priscus in Sigé, p.261–265, Pl. 1 fig. 3–5
1981: Rhinolophus cf. priscus in Crochet et al., tabl. 2-2
1987: Rhinolophus sp. in Remy et al., tabl. 1a, p. 177
1995: Vaylatsia cf. prisca in Legendre et al., p. 64–65
2006a: Vaylatsia sp. A et B in Maitre et al., p. 116–118, fig. 5a
2006: Vaylatsia prisca (pro parte) in Sigé & Crochet, p. 198
Diagnosis: species of intermediate size between V. pelissiei and V. garouillasensis. Rectangular lower molars, with a wide cingulid at the anterolabial corner, and an indented labial cingulid between the trigonid and the talonid. P2/ proportionally smaller than that of V. pelissiei, and less reduced than that of V. Le garouillasensis.
Derivatio nominis: from the Latin frequens: widespread, due to its occurrence in many Quercy localities, so spanning a wide period of time.
Holotype: ESCC VafA.3.21, right hemimandible with the alveoli of I/1–2, C/1 and bearing P/4–M/1–2–3 (Plate 24f), from the UM2 collections.
Type-locality: Escamps (MP 19), Lot, Phosphorites du Quercy, France. Other localities: Caterpillar (Eo/Oligo. indet.), Glaudys (upp. Eo. indet.), Ginouillac, StAntonin-Noble-Val, Clapassou (MP 17a), Perrière, Malpérié, Coyrou 3 (MP 17b), Théron, Monteils, Gousnat, Sindou D, Ste-Néboule (MP 18), Guirolle Rouge, Rosières 1–2–4, Coânac 1, Célarié ocre, Célarié Standard, Lostange, Guirolle Blanc (MP 19), Pécarel, Tabarly (MP 20), Coyrou 1-2 (MP 20/21), Cloup d’Aural 1, (post MP 20), Aubrelong 1, Ravet-Lupo, (MP 21), Lapize (1, 2) (MP 21/22), Pendaré (MP 22), Gardiol 3 (MP 23).
Material and measurements: see Appendix 4.
Description: Mandible: horizontal ramus depth less than the height of one molar crown; mental fossa located below P/2; low ascending ramus, straightening gradually posterior to M/3, then subvertical, with a slightly elevated base in relation to the horizontal ramus; reduced masseteric surface; much extended angular process, facing posteriorly and laterally (Fig. 26b).
Two incisors, as indicated only by the alveoli anterior to C/1.
C/1 typical of the genus Vaylatsia, surrounded by a cingulid, raised anteriorly, growing much wider lingually and posteriorly; wide sinus between the lamella, turned lingually, and the posterior edge.
P/2 almost as long and tall as P/4; oval occlusal shape, sometimes a little wider posteriorly, slightly pinched anteriorly and posteriorly; surrounded by a cingulid, thickening lingually.
P/3 vestigial, comparatively reduced, leaning towards the labial edge of the jaw.
P/4 with a protoconid and a metaconid, rudimentary in nature; surrounded by pronounced cingulid, widening strongly anteriorly, flanked by a sinusid, and widening posteriorly.
M/1–2 with a rectangular shape; thick precingulid; open trigonid, mainly on M/1; developed talonid; sinuous pre-entocristid; raised entoconid; cuspidate hypoconulid, leaning slightly posteriorly.
M/3 slightly smaller than M/2; less open trigonid; slightly narrower trigonid; and bearing a distinct hypoconulid.
C1/ with a sinuous labial edge, strongly raised anteriorly; wide lingual ridge, very thick anteriorly, and ending in a projection on the labial aspect; lamella very curved posteriorly.
P2/ with two very close roots; oval occlusal contour.
P4/ transversal; composed of a raised paracone with a prominent postparacrista, a parastyle projecting from the anterior edge of the tooth, a protocone positioned on the crest connecting the paracone with the heel cingulum, slightly cuspidate; heel very developed posterolingually and with an extended basin.
M1–2/ with an extended protofossa, closed by the connections between the preprotocrista and the precingulum, and postprotocrista and postcingulum; short protocone; developed protocone and slightly projecting mesostyle on labial edge; internal ectoloph branches as long as external branches; significant variability of the heel: more or less extended posterolingually, distinctly more so on M1/ than on M2/; relatively thick cingulum at posterior extremity, sometimes developing as an incipient hypocone.
M3/ less reduced; generally three ectoloph branches, sometimes four (thus creating a larger projection on the posterior edge of the tooth); extended protofossa, closed in the same manner as on M1–2/; labial edge of the tooth less oblique.
Comparison: the population from Coyrou 3 has P/4 with very indented edges and lingually bilobed P4/, strongly pinched at the junction between the heel cingulum and the postcingulum. The size of V. frequens nov. sp. increases from Célarié ocre to Coânac 1, then to Rosières1, 2, 4, and then to Escamps. At reference level MP 21, the population from Ravet displays a larger size per individual than that of Aubrelong 1.
V. frequens nov. sp. is distinctly larger than V. pelissiei nov. sp., its P/4 with wider cingulid; its lower molars are more rectangular with a widened cingulid at the anterolabial corner; trigonid and talonid are clearly separated from one another by a wide indentation of the labial cingulid; P2/ is proportionally smaller, and M3/ longer.
V. garouillasensis is larger than V. frequens nov. sp., and it has a smaller P2/.
Vaylatsia cregolensis nov. sp. (Plate 24j–m)
Synonymy: 1979: Rhinolophus antiquus in Sigé et al., p. 47, 88
Diagnosis: small species of Vaylatsia; very small P/3; long and narrow P/4; wide M2/ with developed heel bearing a wide cingulum; M3/ relatively unreduced.
Derivatio nominis: from the name of the type-locality, Crégols (entrance on western slope).
Holotype: CRE VasA.1.1, fragment of right hemimandible with the alveoli of C/1, P/2, P/3 and bearing P/4–M/2 (Plate 24j), from the UM2 collections.
Type-locality: Crégols (MP 18), Lot, Phosphorites du Quercy, France.
Other localities: St-Lizier, Lavergne (MP 16), Perrière (MP 17b), Sindou D (MP 18), Rosières 4 (MP 19).
Material and measurements: see Appendix 4.
Description: P/2 monoradiculate/with single root, as indicated by an alveolus posterior to C/1.
P/3 vestigial; root deviating towards the labial edge of the jaw.
P/4 narrow and long; dominant protoconid; rudimentary metaconid; concave anterolingual and posterior aspects; flat labial face; labially sinuous cingulid, rising to a point posteriorly.
Nyctalodont lower molars; hypoconulid separated from the entoconid by a wide sinusid; elongated teeth; wide open trigonid, extended anteriorly and quite narrow on M/1, a little wider on M/2; talonid offset labially with respect to the trigonid; developed entoconid; slightly cuspidate hypoconulid, projecting posteriorly.
M/3 reduced in comparison to M/2; shorter and narrower talonid than trigonid; small hypoconulid, adpressed against the entoconid.
M2/ more transversely extended than M1/; squat protocone; widely extended, closed protofossa; internal branches of the ectoloph as long as external ones, so that the mesostyle projects from the labial edge of the tooth; preprotocrista and postprotocrista connected to the parastyle and the metastyle respectively; developed heel, bearing a strong cingulum.
Comparison: the M1/ from Sindou D seems small in comparison to the lower molars from the same locality, also to the M2/ from La Bouffie, even if its morphology appears to correspond well. The presumed synspecific association of the M2/ from la Bouffie to the lower teeth from the other localities is based on the more complete observation of the V. pelissiei nov. sp. material from Les Pradigues, which, despite a slightly larger size, exhibits upper molars with a similar morphology to La Bouffie ones, as well as lower teeth similar to those from Perrière or Sindou D.
V. pumilia is distinctly larger.
V. astruci nov. sp. is distinctly larger; P/3 appears less reduced; its P/4 shorter and wider; and M/3 with a longer talonid than those of V. cregolensis nov. sp. Finally, this species exhibits upper molars with a much less developed heel, and M3/ that is less reduced and proportionally longer, with four distinct ectoloph branches.
Discussion on the genus Vaylatsia:
In their study of the material from Le Mormont (Switzerland), Hooker and Weidmann (2000) continue to attribute some of the specimens to the fossil and extant genus Rhinolophus, and do not follow Sigé (1990, 1995) in recognizing the genus Vaylatsia. Given what is known of the Quercy assemblages, they state that the dental material alone is not sufficient to permit such an attribution. The present work clearly shows the necessity for this new genus name through the systematic association of this archaic rhinolophid dental morphology and a hipposiderid-type distal humerus with a spherical condyle and its functional implication. Given the quality and quantity of Quercy material, the genus Vaylatsia appears well distinct from the genus Rhinolophus, and thus useful and necessary to the clarification of the Rhinolophoidea systematics. The many species (recently described or new) assigned to this genus and attributable to different lineages ranging from the end of the middle Eocene to the lower Oligocene at least, confirm its validity, and likely demonstrate a close phylogenetic relationship with the genus Rhinolophus.
Superfamily indet. A
Family MIXOPTERYGIDAE MAITRE, 2008
Four species and two genera have been identified and described based upon 600 dental remains from 35 localities. The genera Mixopteryx and Carcinipteryx are found from the Eocene to the Oligocene. They display an original combination of dental characters which are habitually observed independently from each other in emballonurids and hipposiderids. Based on this atypical morphological mix, several specimens had been improperly attributed, left with open nomenclature, or erroneously attributed to the genera Vespertiliavus or Hipposideros (Pseudorhinolophus). The comparative study of a large number of specimens revealed real similarities and characteristics specific to a new group: the Mixopterygidae. With this new palaeontological data, phylogenetic relationships within the order Chiroptera were questioned: with the establishment of Mixopterygidae, Emballonuridae and Hipposideridae appear to derive from a common ancestor dating from the very beginning of the Eocene, supporting the monophyly of Yinochiroptera within the Microchiroptera (see Koopman 1985, 1994; Jones et al. 2002). The details of these results are discussed in Maitre et al. (2008a, b).
Superfamily indet. B
Family indet.
Genus Necromantis WEITHOFER, 1887 (Plate 25)
Necromantis adichaster Weithofer 1887 from old Quercy collections, Rosières 5 and Perrière: a Qu-16369 (cast), left hemimandible with C/1, P/2–4, M/1–2–3. b PRR-71, right C1/, labial view (left) and lingual view (right). c ROS5-51, left M/1. d ROS5_NaA.1.1, left P4/. e PRR-66, left M1/. f PRR-70, left M2/. Necromantis gezei from La Bouffie (Hand et al. 2012): g BFI_NmA.1.1, right M/1. h BFI_NmA.1.4, broken right P4/. i BFI_NmA.1.5, holotype, right M2/. Necromantis marandati from Cuzal (Hand et al. 2012): j CUZ_NgA.1.1, left C1/, labial view (left) and lingual view (right). k CUZ-383, holotype, right M2/
The largest bat species yielded by the Phosphorites du Quercy fillings belong to the genus Necromantis. The type species, Necromantis adichaster, was described on the basis of fragmentary material of indeterminate age, as it originated from the artificially mixed Old Quercy Collections. Based on the same collections, Revilliod (1920) revised this genus by describing the cranial material, and added two new species: N. planifrons and N. grandis. Today, the morphological and size characteristics of the material relating to these two species are considered to be within the range of variability of a single species: N. adichaster. Given its size, Revilliod attributed the genus Necromantis to the palaeotropical family Megadermatidae. The adjunction of material from the Old Quercy Collections as well as specimens of the new collections helps to demonstrate the presence of this genus from the middle Eocene, at least, to the upper Eocene (~36–44 Ma). The dental, cranial and postcranial morphologies suggest that Necromantis adichaster was a large and robust bat suited to predation of tough prey such as small vertebrates, perhaps even being a carrion feeder, as suggested by Weithofer through the name of the taxon. However, new data (two new species described in Hand et al. 2012 and thus not here, N. gezei and N. marandati, but see Plate 25 for specimens attributed to both species) raise questions about its phylogenetic affinities, discussed in more detail in Hand et al. (2012).
Discussion
Results of the taxonomic diversity
Study and revision of the material from almost 90 palaeokarstic localities in western Europe have helped to identify 56 Eocene and Oligocene bat species to date. They are spread across 10 genera and 7 families, from reference level MP 13 (middle Eocene, ~43 Ma) to reference level MP 25 (lower upper Oligocene, ~28 Ma). Twenty-four of these are new and nine are “classic” species, generally described from material that has not been precisely situated (localities of indeterminate origin and geological age). In this work, a reference population is ascribed to the types of these species. This population is well situated in both time and geographic location, and provides data for the taxon that are more extensive in their morphological and size variation. Therefore these old taxa can be reconfirmed in a useful manner (Figs. 28, 29).
Summary of Chiroptera diversity from the middle Eocene to the lower Oligocene in western Europe (Vespertilionidae, Molossidae, Palaeochiropterygidae, and Emballonuridae). Symbols: when a species has subspecies, these are distinguished by the grey circles for the oldest, then black and white circles for the most recent; white squares indicate indeterminate subspecies. For the deposit of Ravet, which has two loci, the initial of each locus is provided: D for Ravet-déblai, L for Ravet-Lupo; reference level MP 25 has several localities of the same age as Le Garouillas, studied by Sigé (1990, 1995) and for which the initials are provided when the species is also found there (B Belgarric, P Pécofi, R Rigal-Jouet, Ph Phalip, P3 La Plante 3, Gg La Garrigue, E L’Escoufle)
Summary of Chiroptera diversity from the middle Eocene to the lower Oligocene in western Europe (Hipposideridae, Mixopterygidae, and Family unknown). See Fig. 28 for the meaning of symbols
Phylogenetic hypotheses and deduced phyletic lineages
Using size and the morphological characteristics described above, it becomes possible to propose relationships between the morphological species of the various families identified in the deposits studied. Most of these evolved based on anagenetic processes, within phyletic lineages, over a relatively long period of time. Some only comprise a single morphological species, others regroup a succession of taxa (chronospecies or chronosubspecies), but all exhibit an evolution in dental morphology and/or an evolution in size. Altogether, 33 lineages have been identified within the 7 known families found in the Quercy palaeokarst and other sites, ranging from reference levels MP 13 to MP 23. Comparisons with the Quercy faunas from reference level MP 25 (Le Garouillas and its contemporaries) help to draw links between the taxonomical identifications stemming from this study and those from more recent reference levels, characterized by a series of new species (Ziegler 2000). The relative significance afforded to information from reference levels MP 20 and MP 21, for which there are few localities, is notable.
Family Vespertilionidae
The paucity of data pertaining to this family and the small amount of material by which it is represented in this study renders further consideration of the phyletic relationships within this ensemble impossible.
Family Molossidae
Genus Cuvierimops (Fig. 30)
The cavernicolous variety of this family seems quite small, with only two phyletic lineages within a single genus: Cuvierimops. One is represented by the undetermined species from Le Garouillas (MP 25), C. sp. A, distinctly smaller than the species already determined that makes up the other established lineage. In principle, it appears to originate from the same ancestor as C. parisiensis priscus, from which it differs by only a few features.
The second lineage is confirmed from reference levels MP 17a to MP 22. It does not show any significant morphological changes during this roughly 8 million year period, except for a simplification of the anterior part of the jaw through anteroposterior compression of the premolars between C. pa. parisiensis and C. legendrei nov. sp. and the appearance and increasing proportion of submyotodonty and subsequently myotodonty on the lower molars from C. pa. priscus to C. pa. intermedius, then C. pa. parisiensis and finally C. legendrei nov. sp. The successive appearance of submyotodonty and the increase in number of morphologically different teeth highlight the evolutionary signal of this character, from nyctalodont teeth to myotodont teeth. These changes are accompanied by an increase in size, as highlighted by comparisons of the average dimensions of the M/1–2 and the M/1 measured for each of the subspecies and species identified (Fig. 31).
This study adds significant additional material to the few specimens of C. parisiensis described in Legendre and Sigé (1982). Thus, our knowledge of the variability that exists within this group can be updated and our understanding of the potential patristic affinities to extant genera can be improved. Firstly, the morphology of the upper molars now clearly shows the relationship between the genera Cuvierimops (fossil) and Rhizomops (extant; phylogenetic relationship discussed by Legendre (1985), with the presence on upper molars of a paraloph and a metaloph, a crestiform hypocone (beginning as early as MP 22 for Cuvierimops legendrei), and a protofossa closed by the postprotocrista.
Similarly, the trend towards myotodont morphology in the lower molars of C. parisiensis and C. legendrei nov. sp., being relatively weak at several reference levels, suggests that this character was not selected during this speciation. It would have been, however, during the speciation of Mormopterus, another extant genus with relatively similar morphology to that of Cuvierimops, which has myotodont lower molars. This character, assumed to be derived, would therefore have become selected after speciation. The presence of distinct paralophs and metalophs and a well-developed hypocone on the upper molars supports this hypothesis. These results are completely in line with those of Legendre (1984a), who suggested this relationship based on comparison of extant and fossil dental material, highlighting the association of ancestral characters (presence of a paraloph and distinct metaloph) with newly acquired characters (myotodonty). The various elements recorded in this study all point to Tadaridinae originating from the old genus Cuvierimops (Fig. 32), as suggested by Legendre (1985).
Possible phylogenetic relationships of the Tadaridinae (the number of branches indicates the number of lineages; modified from Legendre (1985)
Family Palaeochiropterygidae
Genus Stehlinia (Fig. 33)
This genus is the only one representing the Palaeochiropterygidae in the Quercy. With 10 morphological species spread across 6 lineages ranging from the middle Eocene (Aumelas, reference level MP 13) to the early late Oligocene (Le Garouillas, reference level MP 25), the genus Stehlinia seems to be relatively well diversified, even though its abundance in the studied localities is relatively low. Thus, only a few deposits, such as Escamps or Baraval, can be characterized by material dominated by species of this genus, which apparently would not have been strictly reliant on karstic environments and perhaps preferred/inhabited more open environments. The existence of at least 5 lineages as early as reference level MP 13 suggests a much earlier appearance and certainly makes Stehlinia the earliest genus of all those present in the Eocene in the Quercy.
The few specimens present at St-Maximin demonstrate the existence of the species S. pusilla, until now known only by its relatively fragmented type-specimen. The additional specimen illustrates the affinity with the species S. gracilis, from which it has few morphological differences and has a relatively similar size. The latter endures at least until reference level MP 23 in the form of two chronological subspecies, S. gracilis gracilis and S. gr. mutans nov. ssp., following one another somewhere around reference level MP 18. This anagenetic evolution translates into the development of myotodonty, decrease in length (squarer lower molars, wider talonid), and more moderate reduction of M3/.
The presence of a new species, S. alia nov. sp., in three localities at reference level MP 13 (St-Maximin, Cuzal, and Chamblon) helps to identify it as the ancestor of the species S. minor, based on its intermediate size between the gracilis and revilliodi lineages, and how similar their dental morphology is to that of the other species of the genus. From reference level MP 21 onwards, there is a notable increase in size, a simplification tied to the decrease in length of both lower and upper premolar rows and e reduction or even disappearance of the hypoconulid as acuspidate relief on M/3 and of the metaloph on M1–2/. These characterize S. bonisi, a species notably identified at Le Garouillas, and found in deposits that postdate the Grande Coupure (Sigé 1990, 1995).
Two other lineages, represented by S. quercyi (of distinctly larger size) and S. rütimeyeri respectively, seem to have the same ancestor. Only these two species have such elongate lower molars showing a proportionally narrow trigonid.
At reference level MP 13, the species S. revilliodi nov. sp. is identified in two localities (Cuzal, and Chamblon), from material of intermediate size between S. minor and S. quercyi. S. revilliodi nov. sp. appears to be closer to the species from Aubrelong 2, S. sp. B, occurring in the same size range.
Finally, the few specimens from St-Maximin assigned to the species Stehlinia sp. A are incompatible in size and morphology with the other species of Stehlinia identified. Thus, Stehlinia sp. A appears to represent another lineage.
It is important to note that only two of the six established lineages of Stehlinia would seem to have survived the Grande Coupure: the lineage gracilis from reference level MP 19 onwards and the lineage minor from reference level MP 21 onwards. Each of these lineages presents distinct morphological variations.
Family Emballonuridae
Genus Vespertiliavus (Fig. 34)
Vespertiliavus is a well-diversified genus in the karstic deposits of the Quercy, with the identification of 9 well-documented morphological species found as early as the end of the middle Eocene. Generally frequent and abundant in the localities studied, it is possible to define 6 phyletic lineages stretching from reference levels MP 13 to MP 25. These are mainly defined by size and generally characterized by a strong morphological variability.
Firstly, the species V. bourguignati, only known until now by the type-specimen from the Old Quercy Collections, is now identified at La Bouffie (MP 17a). It was impossible to group with any other species and it represents a separate lineage.
The existence of another large-sized lineage, V. wingei, is also only indicated by the species of the same name. Being distinctly larger than V. bourguignati, this unlikely to correspond to the evolution of the same lineage. V. wingei is only found in two localities at reference levels MP 17b and MP 19.
The lineage schlosseri, more common and of slightly smaller size than the lineage bourguignati, is identified from a single species, V. schlosseri, from reference levels MP 16 to MP 19. The submyotodont structure of the lower molars begins in this lineage, whilst remaining quite rare based on the material available.
The species V. gracilis, identified from reference levels MP 16 to MP 23, is very similar in size and morphology to V. gerscheli, a species present as early as MP 25. The significant variability of the structural form of the lower molars and the heel of the upper molars of V. gracilis seems congruent with that observed in the teeth of V. gerscheli.
This lineage is very conservative, both in size and morphology, as V. gracilis spans more than 8 million years and crosses the Grande Coupure without apparent change. The strong variability observed across the dental categories illustrates the morphological plasticity of this taxon, and its capacity for adaptation to environmental changes.
V. lapradensis, a species smaller than V. gracilis and found at reference levels MP 13 and MP 14, appears morphologically closer to V. disjunctus nov. sp., with the trigonid of the lower molars less transversely extended and less open, and the talonid of M/3 more reduced than on V. gracilis. Indeed, the difference in size is greater in comparison to V. disjunctus nov. sp. than it is to V. gracilis, but this increase in size is also found throughout the evolution of the lineage, with the distinction of two chronosubspecies using size as a criterion: V. di. disjunctus for reference levels before MP 20, and V. di. nauzensis nov. ssp. for the more recent reference levels (Fig. 35).
Finally, within the new subgenus Sigeia, the lineage lizierensis is not only distinctly smaller than the other lineages of the genus but also the only one to stand out so clearly by its morphological characteristics. Despite the time interval between the occurrences of the two species assigned to Sigeia nov. sgen. being relatively long, this lineage may group V. (Si.) lizierensis nov. sgen., nov. sp. and V. (Si.) recens nov. sgen., nov. sp. Different in size, they both have very peculiar dental morphologies. The oldest species, V. (Si.) lizierensis nov. sgen., nov. sp., is known from reference level MP 16 to reference level MP 17b, and represents the ancestor of the species V. (Si.) recens nov. sgen., nov. sp., the presence of which is demonstrated at reference level MP 22.
Of all lineages considered, half appears not to have survived the Grande Coupure, whereas the others seem relatively conservative, at least in regards to their dental morphology. The strong diversification of the genus Vespertiliavus as early as reference level MP 16, and the presence of a species as early as reference level MP 13, would seem to imply an even earlier origin for this ensemble.
Family Hipposideridae
The family Hipposideridae is the most abundant family of bats found in the Quercy at that time. It includes 18 named morphological species, divided into three genera from the middle Eocene to the end of the lower Oligocene. This family managed to colonize many ecological niches with a range of sizes, from the smallest observed in this study (~7 g) to the largest (~37 g, Necromantis excepted).
Genus Hipposideros (Pseudorhinolophus) (Fig. 36)
This genus, frequently observed in large numbers, is relatively well documented from reference levels MP 16 MP 25 (and beyond that into the Neogene), with an early appearance at reference level MP 13 (Chamblon). The six separate morphological species seem to be organized into four distinct lineages, based mainly on their size. These are generally highly variable in regards to the development and form of the heel of the upper molars. The potential for a fifth lineage exists due to the presence of a still unidentified Hipposideros species at reference level MP 13.
The species H. (Ps.) morloti, known by two morphologically very similar chronosubspecies, H. (Ps.) mo. morloti and H. (Ps.) mo. sequens nov. ssp., represents a single phyletic lineage ranging from reference levels MP 16 to MP 23, characterized by an increase in size over time. The subspecies identification is based on the large size, the opening of the protofossa on M2/, and the heel being less extended on P4/ and M1–2/ of the more recent subspecies. H. (Ps.) mo. sequens nov. ssp. appears around the Grande Coupure after an episode when H. (Ps.) mo. morloti was highly abundant (MP 16 to MP 17b), followed by its quasi-absence (MP 18 to MP 21).
The evolution of the lineage schlosseri before the Grande Coupure, is relatively well described, having been present at almost all of the localities, but appears only sporadically at reference level MP 22, and then disappear. It is identified by two subspecies, H. (Ps.) sc. salemensis nov. ssp. and H. (Ps.) sc. schlosseri, from reference level MP 18 and characterized by an increase in size (Fig. 37) and reduction of P2/2.
The lineage russelli is of large size, characterized by the succession of species H. (Ps.) major nov. sp. and H. (Ps.) russelli nov. sp. It stands out from other lineages for not only its size but also its morphology, notably due to the strong reduction of M3/3, which continues over time. Its existence is documented from reference levels MP 17a to MP 22, with subspecific evolution at Ste-Néboule, right at the end of reference level MP 18, thus well before the Grande Coupure.
Lastly, the subgenus H. Pseudorhinolophus is also represented by two species of very small size grouped within a single lineage. Its presence is demonstrated from reference levels MP 16 to MP 25, where it is the only lineage to remain, despite being generally scattered and not very abundant based upon the material observed. The relatively frequent occurrence of H. (Ps.) tenuis nov. sp. at reference level MP 19, is followed by a period with no trace in the fossil record, and then by the appearance of the species H. (Ps.) zbrjdi, found in reference level MP 25 localities (Sigé 1990, 1995) and apparently a descendant of the small species observed in the localities studied. This evolutionary replacement could have taken place around the time of the Grande Coupure, as for several other species of bats.
The strong diversity already found at reference level MP 16 most likely implies a much earlier appearance of this genus, early enough at least to allow such diversification. The few specimens from reference level MP 13 (Chamblon) suggest an appearance close to that time.
Genus Palaeophyllophora (Fig. 38)
This genus regroups species of average and large size spread across five lineages, from reference levels MP 13 to MP 23. They generally exhibit a strong variability of the premolars (presence/absence of vestigial P/3, variation of the occlusal contour of P4/4), and the molars (relatively median position of hypoconulid, development of parastyle and heel, length of M3/).
The lineage oltina, from the name of the classic species P. oltina, only found in the locality of Ste-Néboule (MP 18), may be the descendant of an as-yet-unnamed species, P. sp. B, found at Cuzal (MP 13). The small amount of information available for comparison does not guarantee this hypothesis but the large size and good morphological correspondence of the few available specimens support this proposal. The presence of Palaeophyllophora as early as reference level MP 13 confirms the existence of the family Hipposideridae at this time. This could have been predicted in light of the strong diversity by which the family is characterized already at reference level MP 17a.
The species P. quercyi, known from the beginning of palaeontological studies in the Quercy, even though only properly determined later, is observed from MP 16 to MP 19. P. nova nov. sp., a species of similar size but with a much more atypical dental morphology for this genus, appears to follow on from MP 20, lasting until MP 23. This rapid anagenesis, mainly involving reduction of the talonid and the protofossa, took place at the time of the Grande Coupure. Given the common occurrence of the new species P. nova. nov. sp., this anagenetic event seems to have been a strong evolutionary success.
Another lineage can be identified from a single species, P. parva nov. sp., morphologically very similar to P. quercyi, but different due to its smaller size. It is found from MP 17a to MP 22, without any notable evolutionary modifications.
The lineage rosierensis can be proposed for a new species of intermediate size between the lineages oltina and quercyi. It comprises only a single species of the same name, which spans from MP 17a to MP 22. It is important to note the smaller representation of this taxon in post-Grande Coupure localities.
There is potential for a last lineage for the species presumed to belong to the genus Palaeophyllophora at St-Maximin, but this remains highly speculative, given the small amount of material available. This second presence of the genus at reference level MP 13 supports the hypothesis of an old age for the origin of this genus.
Genus Vaylatsia (Fig. 39)
Recognition of species diversity in this genus has clearly increased with this study, with the determination of four new species out of a total of seven. It is represented from the beginning of the upper Eocene and until the end of the lower Oligocene by four lineages, separated by size and morphology.
The species V. astruci nov. sp. is frequent from reference levels MP 16 to MP 17b, then becomes rarer until its apparent replacement sometime around the Grande Coupure by the species V. pelissiei nov. sp., which is larger, with longer M3/3 and more transversely developed upper dentition. This lineage is found until reference level MP 23.
A very large-sized lineage is identifiable at reference levels MP 19 and MP 20, from the presence of the species V. valettei nov. sp. in two localities. The absence of this same species in the more recent reference levels suggests that it disappeared, at least regionally, sometime around the Grande Coupure.
The distinction of the new species V. frequens nov. sp., due to its larger size than the other species of the lineage as well as its strongly reduced posterior dentition (M3/3), supports the separation of a different lineage, found from reference levels MP 17a to MP 25. At that point, the species V. frequens nov. sp. gives way to a species of larger size and more reduced P2/, V. garouillasensis. The relative abundance of this lineage increases as that of lineage pelissiei wanes. Confusion in determination between the two species may lead to this type of situation but the differences in morphology and size are clear enough that error risk is minimal. These results suggest these two lineages were in competition within the same ecological niche with V. frequens nov. sp., which was very common in localities of the end of the Eocene. They fragilized during the Grande Coupure, with far fewer occurrences after reference level MP 20, whereas the lineage pelissiei seems less affected.
A lineage of small size regroups a succession of species from reference levels MP 16 to MP 25. Relatively uncommon, V. cregolensis nov. sp. approached the Grande Coupure without showing signs of waning, but seems to have been replaced by a larger species, V. pumilio, already known before this study at reference level MP 25 and today found as early as reference level MP 21.
Family Mixopterygidae
The phyletic lineages inferred for the species of the two genera assigned to this family are described in Maitre et al. (2008a, b). It can be further said that the end-Eocene events appear to have affected the genus Carcinipteryx to the point of a potential extinction of the taxon, whereas Mixopteryx seems to have diversified.
Undetermined family
Genus Necromantis (Fig. 40)
The relatively small amount of material and limited diversity of this bat restrict phyletic interpretations. It would seem however that two lineages can be distinguished within this generic ensemble found from the middle Eocene (reference level MP 13) to the upper Eocene (reference level MP 17b), as discussed in Hand et al. (2012). The speciation of N. adichaster from N. marandati Maitre, in Hand et al. (2012), appears probable given the different elements. The size and general structure of the lower molars remain the same whereas the protofossa opens on the upper molars. It is important to note that this type of morphological evolution is also observed within the lineages of Carcinipteryx (Mixopterygidae) and that it is another example of the phenomenon of mosaic evolution, already considered in Maitre et al. (2008a, b).
As to the species N. gezei Maitre, in Hand et al. (2012), from reference level MP 16, it defines a second lineage of distinctly smaller size but similar morphological characters to those of N. marandati, namely still underived compared to the more evolved type of N. adichaster. As in the genus Carcinipteryx, the morphological anagenetic evolution may be the same for both lineages.
Evolutionary synthesis of the families studied
Based on their dental morphology, the different families of bats found in western Europe in the Eocene were not all affected by the transition towards the Oligocene in the same way, being constrained by various factors (palaeoclimatic, palaeoenvironmental or pertaining to biogeographic distribution). Some show no major changes, at most anagenetic evolution. This is likely the case of the Molossidae, with the substitution of Cuvierimops parisiensis parisiensis by C. legendrei nov. sp. from reference level MP 22, and the existence of a new lineage at reference level MP 25.5 Ma after the Grande Coupure. This is also likely the case for the subgenus Hipposideros (Pseudorhinolophus) with continuation of the four lineages throughout reference levels MP 17a to MP 22, even though one of them appears less frequently. The transition would thus be seen in changes that are subspecific or specific to the reference levels preceding the Grande Coupure (MP 18, MP 19 or MP 20): H. (Ps.) morloti sequens nov. ssp., H.(Ps.) schlosseri schlosseri, H. (Ps.) major nov. sp., and H. (Ps.) zbrjdi.
Other taxa are better indicators of environmental changes, such as the genera Palaeophyllophora or Vaylatsia, in which each lose a lineage before the beginning of the Oligocene, and show specific replacements at reference levels MP 20 and 21.
The Palaeochiropterygidae and Emballonuridae appear to be more affected as they lose respectively four and three lineages of the six present before MP 20 at the Eocene–Oligocene transition.
Finally, within the Mixopterygidae, a genus (2 lineages) disappears completely whilst a new lineage appears in the other genus at reference level MP 22. Nevertheless, these conclusions as to the impact of the Grande Coupure on the evolutionary dynamics of bat lineages must be mitigated by the relatively small number of localities, and therefore potential lack of information, for reference levels MP 20 and MP 21.
General phylogeny of Chiroptera (Fig. 41)
The hypothesis of the relationship between Emballonuridae and Hipposideridae, which appear around the same time in the fossil record, is strengthened by their supposed ascendance in common with the Mixopterygidae. The supposed separation of this superfamily from the more archaic Chiroptera helps to date the last common ancestor of these three families. The first known members of this superfamily are those of the Emballonuridae and the Hipposideridae with, respectively, those from Messel (MP 11, Tachypteron franzeni; Storch et al. 2002) and from Chambi (MP 11, Rhinolophoïdea indet.; Sigé, 1991), of which the age was recently provided by Adaci et al. (2007). The supposed common ancestor would thus probably have appeared in the lower Eocene. It is to this first phylogenetically similar ensemble that the genus Necromantis would belong, perhaps associated with the genus Vespertiliavus, or more directly to the archaic group Archeopterygidae.
A second ensemble, which can be grouped under the superfamily of Vespertilionoidea, begins to emerge within the bats observed in the Quercy. It is composed of the Palaeochiropterygidae (Stehlinia), Molossidae and Vespertilionidae. The lack of information regarding tree-dwelling forms, which should notably have included the family Vespertilionidae (Leuconoe), makes it impossible to provide more detail than already previously discussed. More certainly, the Molossidae separated from the Palaeochiropterygidae, with a first appearance at reference level MP 17a (Lébratières 1). The genus Stehlinia must have separated earlier in the lower middle Eocene given the significant diversification shown in several localities at reference level MP 13.
The study of the faunas of more open environments (non-karstic) will help to complete the data, providing more details as to relationships and dating the appearances of the different groups.
Biochronology
The exceptional quantity of fossil material considered in this study, collected from almost 90 localities spanning more than 10 million years, paints an unprecedented picture of the evolutionary history of Palaeogene bats in western Europe. Not only does it mean a considerable reevaluation of the taxonomic diversity and the variability of the dental morphology of this group for each of the reference levels catalogued, but it also provides a highly detailed vision of their evolution over time, through the rapid succession of fossil records spanning a long period of time. Potential anagenetic relationships are identified between the different morphological species based on evolutionary trends and the general observation of within-lineage size increases over time. Successive chronospecies emerge from these phyletic lineages and are characterized by a certain morphology and size.
The co-occurrence of several of these chronospecies within a single fossiliferous deposit thus helps to date it in relation to the other deposits, notably through observing the increase in size amongst a large group of lineages as time passes. This is based on Cope’s Law, which indicates that the average body size in a community of species can only increase over time. Indeed Legendre and Escarguel (2006) state that for the Quercy and Limagne faunas from the upper Eocene to the lower Miocene (bats excepted), the body size of the identified lineages increases in 53 % of cases, decreases in 18 % and remains stable in 29 % (Table 2). These observations serve to push back the age of localities where the species under consideration are smaller. The systematic measurement of all materials observed and well preserved enough (Appendix 4) does indeed illustrate how much more chronological details can be provided to the different faunas, given sufficient population data.
The reference stages
First, the fauna of deposits dated by Escarguel et al. (1997), based on the whole mammal faunas present in these localities, except bats was compared. Chronological information obtained from bats corroborates the dating of reference levels MP 13, 16 and 17a. The species present both at Perrière and Malpérié are generally of similar body size and do not help to date these two deposits. Vespertiliavus gracilis and H. (Pseudorhinolophus) schlosseri salemensis nov. ssp., both well represented in these two localities (see measurement tables, Appendix 4 and summary in Appendix 5), are clearly at their largest at Malpérié. Given the confidence intervals, the earlier age of Perrière does not contradict the numerical age data. However, the fauna of Rosières 5, of a distinctly earlier associated numerical age, has populations of larger body size than the same species at Perrière and smaller body size than that at Malpérié.
At reference level MP 18, the three localities with known numerical ages are Gousnat, Sindou D and Ste-Néboule. Given new information from the study of bats, in accordance with the numerical ages of these localities, Ste-Néboule is shown to be the most recent deposit whereas the other two cannot be distinguished. The presence of Hipposideros (Pseudorhinolophus) major nov. sp. at Ste-Néboule, a replacement that is more noticeable at reference level MP 19 for H. (Ps.) russelli nov. sp. within the lineage of the same name, suggests that this deposit represents the most recent chronological stage to be attributed to reference level MP 18.
The faunas of established numerical ages associated with reference level MP 19 are characterized by body size compatible with their relative chronologies. However, several species found at Escamps appear to be smaller than expected (e.g. Vaylatsia frequens nov. sp., Stehlinia minor, Hipposideros (Pseudorhinolophus) schlosseri schlosseri), and consequently the Rosières 1 deposit seems slightly older than Rosières 2, and hence Rosières 4.
In the lower Oligocene, two populations from Ravet exhibit a larger body size than that of Aubrelong 1. The relative ages of La Plante 2 and of Mas de Got are not easy to determine. Two species suggest that La Plante 2 is older (Vespertiliavus gracilis and Stehlinia gracilis mutans nov. ssp.), but several other species do not give such clear chronological information [Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp., Palaeophyllophora parva nov. sp., Palaeophyllophora nova nov. sp., or Vaylatsia prisca].
These faunas, well documented and studied as a whole, correspond to relatively well-restricted stages in the evolution of the body size and dental morphology of bats. These reference stages of the biochronological scale are established or further clarified here. The relative chronology of the other faunas will be established based on the comparison of these two evolutionary criteria as they apply to the various species these faunas have in common.
Localities with no associated numerical age
Localities with recently studied faunal content
Of the localities of hitherto undetermined numerical age, six (Pépénut, St-Antonin-N.-V., Bouziès, Liauzu, Théron, Monteils) have been the subject of a preliminary study of all faunal groups, bats included (Maitre et al. 2006). The results presented in that paper clearly preceded the completion of the present work. The names indicated for the various species, and often left as open nomenclature, are used in the synonymy lists of this paper. The reference levels to which they are assigned in Maitre et al. (2006) are still in good agreement with the conclusion presented here and can be listed as follows:
-
1.
St-Antonin-Noble-Val, Bouziès: MP 17a
-
2.
Pépénut: MP 17b
-
3.
Liauzu, Monteils, Théron: MP 18
The populations of Baraval (Sigé et al. 1998; Le Gall 2001) and Gardiol 3 (Maitre 2003) have also been the subject of a complete systematic and chronological study of their fauna and are attributed to reference levels MP 22 and MP 23, respectively.
Localities newly studied in this work or as yet unstudied localities
These can be assigned to a reference level based upon the co-occurrence of several bat lineages. Localities such as Lapize (1, 2), where the presence of Hipposideros (Pseudorhinolophus) morloti morloti imposes a date prior to reference level MP 20 and Hipposideros (Pseudorhinolophus) major nov. sp. a date after the end of reference level MP 18, thus pointing to reference level MP 19. Similarly, the deposit at Lébratières 2 is more recent than reference level MP 19 because of the presence of Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp. and later than reference level MP 20 because of Palaeophyllophora nova nov. sp., as are the faunas of Cloup d’Aural 1, La Couaille, Jammes, Baraval, Cavalé, les Bories, Lébratières 12, 13 and 15, and Gardiol 3. Conversely, the localities where Palaeophyllophora quercyi is found would seem to be older than MP 20: Ginouillac, Bouyssou 2 and Palembert. The Hipposideros (Pseudorhinolophus) major found at Pech Pullé implies a date after reference level MP 18. Finally, at Souhic, the presence of Vespertiliavus disjunctus bretouensis nov. sp. and Hipposideros (Pseudorhinolophus) schlosseri schlosseri points to a possible age somewhere between MP 18 and MP 19.
Relative chronology of localities assigned to the same reference levels
More details about the relative ages of different populations tied to the same reference level can be obtained from studying the increase in body size over time for several contemporary lineages (Appendix 5).
Firstly, the locality of Chamblon (MP 13) appears to be more recent than Cuzal, based on the specimens assigned to S. revilliodi nov. sp.
The chronological order of the populations from reference level MP 17a can be determined mainly by the body size of three generally abundant species, found in the largest number of localities: Hipposideros (Pseudorhinolophus) schlosseri salemensis, H. (Ps.) morloti morloti, and P. quercyi. It proves to be that Lébratières 1 and Ginouillac populations are much smaller, and thus possibly older than the others, even if the order of their succession cannot be established. Next, the locality of Tufal also appears smaller than the others without, however, being compared to the two previous localities as they have no taxa in common. Then comes La Bouffie which appears very similar to Salème and St-Antonin-N.-V., Aubrelong 2, Trifon, Les Pradigues and Bouziès. The fauna of Clapassou, which had not yet been studied, provides a good representation of the species found there [Stehlinia minor, Stehlinia gracilis gracilis, Vespertiliavus gracilis, Hipposideros (Pseudorhinolophus) russelli nov. sp., Paleophyllophora quercyi, Vaylatsia frequens nov. sp., and Mixopteryx perrierensis]. Based on the body sizes of its bats, the deposit’s age seems to be relatively well determined and more recent than that of Les Pradigues and Bouziès, whilst still being older than Malpérié and Perrière. Therefore, it seems that this fauna is the most recent of those assigned to reference level MP 17a.
The only tooth found at Coustal does not provide further clarification as to its age, assigned by a succinct analysis of the fauna to reference levels MP 17a or MP 17b.
The fauna of Coyrou 3 can likely be assigned to MP 17b, with an intermediate age between Malpérié and Pépénut, based on the body sizes of the species found at this locality. The body sizes of V. astruci nov. sp. and Hipposideros (Pseudorhinolophus) morloti morloti found there are smaller than those found at Sorcières.
Pech d’Isabeau and then Mas de Bonhomme do not provide much material for comparison but seem to date later than Sorcières. The few species found at the localities of Bouyssou 3 and 2 reveal their biochronological proximity and are close in body size to that species observed at the end of reference levels MP 17b—beginning of MP 18, with Bouyssou 2 being assigned to reference level MP 18 and Bouyssou 3 to reference level MP 17b.
Mixopteryx weithoferi and Cuvierimops parisiensis intermedius nov. ssp., which are the most well-documented species from the locality Théron, clearly indicate a basal position amongst localities assigned to reference level MP 18. The only species found in the Liauzu deposit is a bat, Palaeophyllophora rosierensis nov. sp. The quality of the record helps to suggest a slightly earlier age than for the deposit at Mémerlin-Muséum. The relative age of the populations of Mémerlin-Muséum, Mas de Labat 1 and Crégols can be determined by comparing the body sizes of four species [Hipposideros (Pseudorhinolophus) schlosseri schlosseri, Hipposideros (Pseudorhinolophus) russelli nov. sp., Palaeophyllophora parva nov. sp., and Palaeophyllophora rosierensis nov. sp.], when these are well documented. The fauna of Mas de Labat 1 seems to be more recent than that of Mémerlin-Muséum, which in turn seems more recent than that of Crégols. The specimens from Monteils being in short supply, it is only possible to note that they pre-date those observed at Gousnat, as well as those from deposits previously mentioned for reference level MP 18. The population of Hipposideros (Pseudorhinolophus) schlosseri schlosseri from Trémouls appears to be of intermediate size between that of more recent faunas assigned to reference level MP 18 and the oldest faunas assigned to MP 19, without being possible to be more precise.
Despite the taxonomic and geographic proximity of the faunas of Guirolle Rouge and Guirolle Blanc, the body sizes of their common species indicate that the relative age of the latter is much more recent. These two localities represent the oldest stage and the most recent stage of the faunas from reference level MP 19, respectively. Nougayrac is a fauna with species of clearly intermediate size between the Rosières and the more recent faunas of Guirolle Blanc and Lostange. The species common to both Célarié Standard and Célarié Ocre are very similar in body size, making it impossible to situate one in relation to the other. Clearly, the population of Hipposideros (Pseudorhinolophus) schlosseri from Lostange appears to be of intermediate body size and therefore intermediate age between that of Célarié and Guirolle Blanc. Palembert cannot be more precisely dated than the initial proposition, given the lack of available material.
The species at Pécarel [Stehlinia gracilis mutans nov. ssp., Stehlinia minor, Hipposideros (Pseudorhinolophus) schlosseri schlosseri, Palaeophyllophora rosierensis nov. sp., Vaylatsia frequens nov. sp.] are generally larger than those from the faunas of reference level MP 19, whilst still being noticeably smaller than that of Tabarly. Coyrou 1-2 appears to be closer to reference level MP 21 than the two deposits previously mentioned. However, the small quantity of material available makes it impossible to definitively assign it to reference level MP 20 or 21. The few specimens from Escabasse 2 remain relatively large examples of the species to which they belong, whilst still being generally smaller than those found at Plante 2. It appears reasonable to assign this deposit to reference level MP 21, whilst placing its likely age as more recent than Ravet.
Reference level MP 21, initially proposed for the deposit of Garrhan based on its rodents, cannot be revised here given the single specimen of bats observed in this fauna. The large size of this tooth may point to the age indicated by the rest of the fauna.
The species from Le Mas de Labat 2 [Hipposideros (Pseudorhinolophus) major nov. sp., Palaeophyllophora parva nov. sp., Palaeophyllophora rosierensis nov. sp.] appear to be of intermediate size between that of the corresponding taxa from Tabarly and the faunas assigned to reference level MP 22.
The small locality of Souhic may correspond to a rather recent stage of the lineage Vespertiliavus disjunctus, with a form in line with reference levels MP 21/22. The deposits of Lapize (1, 2) and La Rode appear to be very close in age. Average body size of the species found there [Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp., Hipposideros (Pseudorhinolophus) major nov. sp., Vaylatsia frequens nov. sp.] is always quite large, still being larger than those at reference level MP 21 and smaller than those at reference level MP 22. The same can be said for Pech Pullé judging from the few specimens of Hipposideros (Pseudorhinolophus) major nov. sp.
The deposit of La Couaille presents a fauna typical of the lower Oligocene, likely the oldest deposit to be assigned to reference level MP 22. The population of Jammes would appear to be even more recent, dated to an age intermediate between those of Lébratières 12–13–15 and Mas de Got.
The species from Baraval [amongst them Stehlinia bonisi, Vespertiliavus gracilis, Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp., Palaeophyllophora nova nov. sp.] are of intermediate size between those observed at La Plante 2 and Lébratières 12, 13, and 15, whilst still being smaller than at Cavalé. However, Stehlinia gracilis mutans nov. ssp. and Vaylatsia frequens nov. sp., which are both bigger at Baraval than at Cavalé, make it impossible to establish the relative ages of these two localities. The few specimens from La Nauze 1 would seem to point to an age younger than that of this locality but older than that of Lébratières 15.
Not only do the three deposits of the Lébratières (12, 13, 15) have very few species in common [Hipposideros (Pseudorhinolophus) morloti sequens nov. ssp. and Palaeophyllophora nova nov. sp.], but they also do not have much material and are thus not easily comparable. They are undoubtedly of similar age and correspond to the most recent records assigned to reference level MP 22. The few specimens from Pendaré are characteristics of this same reference level but the relative age with regard to the other faunas of the reference level is hard to specify.
Finally, Gardiol 3 appears to be the most recent deposit of those studied in this work, at an intermediate stage in regard to body size and morphology between the faunas of reference level MP 22 and those, already studied, from reference level MP 25 (Sigé 1990, 1995).
Localities of undetermined age
Glaudys cannot be precisely placed in the upper Eocene given the small number of specimens available. The age of the bats fauna from Bouyssou 1 cannot be evaluated given the small number of specimens available and the almost incompatible forms of the two species represented. The same goes for Caterpillar, which has only provided a single bat specimen.
Localities with multiple fillings
For some localities (notably Escamps and Mas de Got), the material was studied from several samples that correspond either to different karstic fillings that were relatively close to one another and belonged to the same pocket (Mas de Got A, B) or to different stratigraphical levels of the same body of sediments (Escamps A, B, C). It proves to be that there is no difference in morphology and/or body size by which to differentiate them. Only differences in the presence of certain taxa make it possible to distinguish them, but these always correspond to the least abundant species.
The same can be said for the deposit of Baraval, where the distinct stratification of the filling of this small cave made it possible to draw samples and conduct a level-by-level study. The only observable difference was the taxonomic variation in the presence/absence and relative abundance, without noticeable changes in the morphology or size of the specimens. Despite the relatively small number of specimens, the study by Le Gall (2001) on the entire fauna attempted to compare the different levels. The study was only able to highlight a simple ecological variation (abundance or presence/absence) according to intervals that were chronologically evaluated based on sedimentary rhythms. These observations point to a total timespan covered by this filling shorter than 200–400,000 years (Aguilar and Michaux 1997; Escarguel et al. 1997; Escarguel 1998).
The entire biochronological scale obtained here, in this study, is employed in the different figures where chronological succession of localities is needed (Figs. 28, 29, 30). The study of other groups (e.g., rodents, artiodactyls) has already led to biochronological scales being established based on the chronospecies found in the same region. However, none of these were built from such a large number of localities and spanning such a large time frame. Comparison of the different bat populations shows the significant potential for resolution improvement that exists there, and this with a group that has long been viewed as relatively uninclined to evolve. The age of some localities remains to be determined, but the excavations that are still ongoing or planned in the Quercy will help to improve our knowledge of the less documented deposits.
Palaeoecology
In the first part of this paper, the study of the dentition highlighted the taxonomical diversity of bats in western Europe from the middle Eocene to the lower Oligocene. Below, I focus on bat body mass to highlight the characteristics and modalities of their evolution.
Body weight
Weight diversity of the studied material
Size is the main parameter to capture the diversity found in the studied fossil material. It is easily interpretable whatever the specimen (bone, tooth), provided attribution is secure. The length and width measurements of the teeth, recorded in Appendix 4, show a gap in the size range of European species drawn from the period studied in this work. The existing relationship between the surface area of M/1 and body weight, used to produce cenograms (see below), serves to better visualize the distribution of body mass within this group. The observed size gap is not as large as that observed in other mammal orders (rodents in particular, potentially artiodactyls), but there is a gap in the range of weights roughly between 5 and 47 g.
Figure 42 provides an idea of intrageneric body weight diversity in these bats. As a rule, the larger the number of species in a genus, the broader the range of size, suggesting that anagenesis tends to diversify not only the morphology but also the body size. Within each genus, it also appears that average and smaller body sizes largely outnumber larger body sizes, with an incomplete range of sizes between the large species and the others, which is characteristic of log-normal distributions. These missing weights vary in terms of values but the gap remains more or less constant, between 6 and 8 g.
Distribution on a logarithmic axis of the average body weights of the species observed in this work within each genus (related species names and numerical data table given in Appendix 6). The weight of each taxon has been calculated based on the observed (when available) or imputed M/1 surface (see text for details)
Cenogram method
Body mass estimates:
The construction of cenograms is another tool for evaluating the variations in chiropteran community weight distribution over time (Valverde 1964, 1967; Legendre 1986). Cenograms are constructed from estimations of the average weight of individuals, based on the strong correlation existing between the size of the teeth and the body weight for mammals (Appendix 6). Coefficients “a” (the slope) and “Log(b)” (the intercept) of the regression line [Log(weight) = a Log(M/1 surface) + Log(b)] of these two parameters have been calculated based on extant species data compiled by S. Legendre (see method in Legendre, 1989): a = 1.096 and Log(b) = 0.770; with R 2 = 0.845.
Regarding the teeth, the choice of the M/1 surface was made given the satisfying results obtained in many studies by the correlation of tooth size and animal weight. This tooth is actually the least variable in its linear dimensions; the logarithm of its surface is generally the least variable of all cheek teeth and has one of the strongest correlations to the weight of the animal. The use of the tooth surface is justified by the fact that these two dimensions in combination, length and width, provide a better estimation of size than either of these measurements taken separately, as well as the fact that its surface is less dependant on its shape (Legendre 1989).
Body mass estimates when no M/1 is available in a given “species sample”:
For each morphological species from each locality, all of the observed dental material in a sufficiently well-preserved state to take accurate measurements has been measured. When the species sample was very dense, the measurements were made on 100 specimens for each dental category. Therefore, when a species is identified on the basis of a damaged tooth, a humerus or a toothless mandible, it does not appear in the database used for biometric analyses. The dental categories retained are P/4, M/1–2–3 and P4/, M1–2–3/.
C1/1 are not included, given the observed phenomena of dimorphism and the difficulties in determination that exist for some taxa. This is the case for the C/1 of the species of the genus Vaylatsia and those of the genus H. (Pseudorhinolophus) where the morphologies are very similar and can only be assigned to genus when they are present in the mandible, which is far more characteristic. Species represented by a single canine are thus not taken into account in the database analysed below.
P/2, for which the size and shape are very variable within a given population, is frequently absent from the fossil record; the high proportion of these absences in the database leads to their elimination. P2/, however, is often absent from the dental formula or, at best, vestigial and thus rarely found in the material presented.
P3/3 are dental categories of small size, varyingly present within the order Chiroptera, be it at the genus, species or even intra-species level, as seen in species of the genus Palaeophyllophora. They are generally vestigial, stylet shaped and very often the only indication of their existence is an alveolus. In the material studied, only the species of the genera Leuconoe, Vespertiliavus and Stehlinia have actual P3/3.
Species of the genus Necromantis, which have been subjected to more in-depth studies (Hand et al. 2012) after the present work was achieved and which are largely represented by material without geographic localization or datation, are not included in the present database.
For each dental category, the averages of the length and width measurements in millimetres are recorded, supplemented by measurements of talonid and trigonid width for the lower molars. These measurements were taken as indicated in Fig. 3. The complete database therefore represents a table (“A”, see Appendix 7) of 417 lines and 22 columns, where each line represents a sample population (i.e., a species in a given locality) and each column represents the length or width for each dental category measured. This database presents missing data pertaining to the absence of certain dental categories from the material sampled for a given population. Typical of the fossil record, this problem immediately eliminates many of the quantitative (univariate or multivariate) analysis methods potentially applicable to such a measurement table. To remedy this, an estimation tool for incomplete multivariate data is applied to this incomplete database. The multiple imputation approach (Allison 2000; Yuan 2001) appears to be a much less statistically biased technique than other approaches such as multiple regression. For this the NORM method and programme (Schafer 1999) were used. This method generates multiple imputations based on a multivariate normal distribution model. The imputation of the missing data is made through three steps as follows. First, via an Expectation–Maximization (EM) algorithm which allows the maximum likelihood estimation of various parameters (averages, variances, covariances or correlations) through iterations between the two following steps:
-
Step E: replacing the missing data by estimations expected in light of the available data in the matrix
-
Step M: updating of the parameters based on the maximum likelihood estimations from step E.
The speed of convergence of this algorithm depends on the quantity of missing data in the matrix: the more missing inputs there are, the longer the convergence will take.
Second, using Markov Chain Monte Carlo (MCMC) methods to generate several equally probable multiple imputations for each missing value.
Third, all of the complete tables obtained after each imputation are compiled to produce a complete data table where each missing value is replaced by the average of the corresponding imputed values. The NORM programme does not perform this compilation; therefore, an IDL programme was created to automate this last step (Escarguel, unpublished).
This multiple imputation method was initially used on the entire table (imputation table A’; Appendix 8). However, the various statistical analyses performed subsequently showed that the imputations skewed the dataset and introduced significant noise rendering it uninterpretable. Given that the robustness of this method decreases as the number of missing values increases, the table was subsequently reduced with regard to the missing data. The incoherent statistical data helped to empirically identify the threshold at which the number of imputed values no longer skews the dataset: in our case a table comprising taxa (lines) for which more than a quarter of the variables are missing returned statistically inconsistent results. The new, reduced table (B; Appendix 9), significantly smaller than table A (see following example), is thus reduced to 282 lines, finally giving a new imputed data: table B’ (Appendix 10).
For each reference level, the average weight of the individuals is estimated based on the average M/1 surface for all the populations at the time. For each specific occurrence where M/1 is available, it can either refer to the average surface of all M/1 or a single surface from a single specimen.
The M/1 surface of a species is then calculated based on the length and width measured directly on the available material. The cenograms constructed in this study for each reference level represent on the ordinate axis the logarithm of the weight in grams estimated for each species, ordered from the largest to the smallest species size along the abscissa. These range from reference level MP 13 to MP 22, reference level MP 23 being restricted here to the study of a single locality (Figs. 43, 44).
Extant cenograms (Appendix 6):
To better understand the type of information found in the cenograms, an initial study of the cenograms of extant bat communities seemed useful before attempting to interpret the cenograms of fossil bat communities. This serves to determine whether the distribution of chiropteran weights varies depending on the type of living environment, as observed for the broader terrestrial mammal community. The environmental context of western Europe at the Eocene–Oligocene transition being characteristic of a tropical environment, the choice was made to examine cenograms of the tropical regions of the Old World, notably African regions. To represent a wide enough range of environments (such as a humid tropical forest, tropical savannah and light forest, tropical savannah and scrub, tropical savannah and desert), the following regions were included: South Africa (Transvaal), Cameroon, Ethiopia, Gabon and Nigeria. The faunal lists as well as the average body weight of the species were obtained from the literature (Transvaal: Rautenbach 1982; Cameroun: Perret and Allen 1956; Ethiopie: Largen et al. 1974; Gabon: CNRS 1963–1969; Nigéria: Happold 1987). This ensemble serves to produce cenograms representing typical vegetation ranging from humid tropical forests to semi-desertic savannah and to be able to associate these with the characteristics of the body weight distribution of chiropteran fauna.
Cenograms of humid tropical forest environments (Sangmelina, Makokou, Obudu–Boshi–Okwangwo) are characterized by an abundance of large species spread along a curve with roughly the same slope as that of the distribution of smaller species. Differences can however be seen in relation to altitude: higher regions have less large species than lower ones. This phenomenon grows more pronounced in more arid regions such as savannahs or light sub-humid forests, where the slope of the curve for large species is steeper due to their distinctly smaller numbers. Desert regions exhibit a strong decrease in diversity in comparison to all of the other types of environment observed. There are therefore strong correlations between the body weight structure of the bat fauna and the environment in which it exists, as already shown for the entire terrestrial mammal community (Legendre 1986, 1987).
Fossil cenograms:
Based on comparisons with extant cenograms and the small number of species represented, the cenograms for reference levels MP 13, MP 16, MP 20 and MP 21 seem biased due to the small number of localities and the small amount of available material for these time periods (Figs. 43, 44). No time will therefore be spent on their interpretation. Several significant points of comparison stand out between the fossil and extant cenograms:
-
1.
The number of bat species composing the fossil faunas cannot be usefully compared to extant faunas, first of all because the fossil record available does not reflect the entirety of the bat fauna that existed at the time, and second because it represents only a fraction of the bat fauna, mainly cave-dwelling. A better analogy could be drawn with the karstic faunas of South-East Asia (notably Vietnam or Laos), but these remain relatively unknown and unstudied (Furey and Racey 2007).
-
2.
The range of body sizes in extant species is wider, whatever the environment (from forests to savannah). This does not take into account the fact that in extant faunas the larger forms (with weight >50 g) are represented by Pteropodidae and the smaller forms (with weight <5 g) by Nycterididae, Rhinolophidae and Vespertilionidae, families that were almost or totally missing from the Quercy faunas of the period around the Grande Coupure. Equivalents for these large species are perhaps present in the fossil faunas of more open environments, still relatively unknown and not studied to date.
-
3.
A break in the distribution, roughly around the 30-g mark, in extant and fossil cenograms separates the larger forms from the rest of the fauna. This break represents a gap of variable size in extant faunas but remains distinctly larger than that found in fossil faunas. In regard to the previous point, the number of larger species is always much greater in extant faunas, with around seven species as opposed to a maximum of two for the fossil faunas studied in this work.
Given these observations, a fossil cenogram for a bat fauna is, as it stands, hard to interpret in terms of environmental evolution because the distribution of the larger weight class is missing, when the extant distribution is so characteristic of each type of environment. The study of deposits which are from more open environments or of more recent age, containing these missing larger species, may help to reach a conclusion regarding environmental variations and the application of the observation of extant faunas to the fossil record.
Despite this, it is possible to note the decrease in slope angle for the distribution from reference level MP 17b onwards to reference level MP 22, with a constantly diminishing range of sizes. Even if this range represented by extant faunas remains the same (except in the case of very restricted intervals for desert environments), it distinctly decreases over time for fossil faunas.
More precisely, it is both the larger and the smaller forms that are more likely to disappear, regardless of the variation in number of species during this period:
-
MP 17a: from 6 to 36 g, 22 species
-
MP 17b: from 6 to 46 g, 20 species
-
MP 18: from 6 to 36 g, 18 species
-
MP 19: from 6.5 to 34.5 g, 21 species,
-
MP 22: from 7.5 to 34.5 g, 18 species, whereas the number of species of average weight increases.
This method nevertheless serves to highlight noticeable variations in the structure of the bat community from the late Eocene/early Oligocene period. Indeed a change in the distribution of weights takes place before the end of the Eocene and appears to still mark reference level MP 22.
Clearly, these observations show that the body weight structure of the chiropteran faunas is a good reflection of the type of environments in which they live. Despite the fact this is not strictly applicable to this study, the relations established between extant cenograms and habitat strongly suggest that the evolution of the body weight structure of the chiropteran community is a direct proxy of the evolution of environmental conditions.
Impact of the Grande Coupure on Chiroptera
The results from the cenogram analysis can be compared to those obtained from the study of dental morphology characters in association with body size changes within phyletic lineages. Together, all these data and results point to a period of relatively significant changes within the bat community at the Eocene–Oligocene transition, be it through a decrease in taxonomic diversity and number of lineages, or through the diversity of tooth shape, and the range or category of weight class/body mass. This period begins as early as reference level MP 18 and lasts until reference level MP 21, potentially even MP 22. It is preceded and followed by phases of strong diversification and anagenetic events, with the occupation of vacant morphological and ecological niches as much with respect to morphology as to tooth shape or weight categories.
Families are not all affected in the same way. The cenograms highlight the disappearance of the “extreme” species. The largest and smallest species seem to disappear preferentially. The modalities and consequence at the community scale are illustrated by the quantitative studies, which show that the disappearance of a species is tied to its specific characteristics and directly affects the structure of the community (decrease in body weight range). Such indicators are interesting for fossil faunas to help better understand the causal relationships in the course of the evolution of a clade.
Qualitative and quantitative analyses of the bat fossil record each provides more details about the evolutionary changes in these small mammals. Morphological evolution within the lineages seems to be relatively moderate and rather progressive based on character variations, but the anagenesis, along with the occasional appearance of some new lineages and the more or less abrupt disappearance of others in a relatively tight timeframe, points to a particular phenomenon.
Chiroptera (at least the cave-dwelling ones), much like other vertebrate groups in western Europe, were also subjected to Stehlin’s (1909) Grande Coupure. Indeed most of the paleontological studies made on this continent have shown the reality of this biogeographic and climatic event, with numerous disappearances recorded amongst the condylartha, primates, creodonts, artiodactyls or even rodents (Sigé 1976; Sigé and Vianey-Liaud 1979; Maitre et al. 2006a, b). A faunal assessment of the mammals and birds of the Massif Centrale (Quercy and Limagnes) highlights high species abundance for reference level MP 19, followed by a decrease at reference level MP 20 (Escarguel and Legendre 2006; Legendre et al. 2006). This phase of environmental changes did not induce immediate reactions but rather an evolutionary phenomenon of progressive modifications spreading over almost 3 million years, for the Chiroptera, with a succession of disappearances and renewals/immigrations.
Conclusions
The study of the bat fossil content of almost 90 localities from the middle Eocene to the early Oligocene time period, for the most part being palaeokarstic and from the same region of the Quercy (southwestern France), makes this study unique to date, providing a chronologically dense and accurate information based on the direct observation, description and systematic comparison of dental material. The palaeobiodiversity of the group was established with the identification of 7 families, 10 genera and 57 species, amongst them several newly proposed taxa (1 subgenus, 17 species and 8 species still in open nomenclature). Several “classic” taxa were identified and associated with properly localized and dated reference populations, providing a notable improvement to the documentation for these taxa and the possibility of determining their biochronological position. These documentary additions are still lacking for reference levels from the middle Eocene and for those surrounding Stehlin’s (1909) Grande Coupure (MP 20–21). This justifies continued excavation in the palaeokarst to increase the amount of data from the known sites and the possible discovery of new deposits (e.g. Maitre 2006a).
The differences in size and morphology highlighted between the taxa have served as a basis for the identification of 33 specific phyletic lineages, the numbers varying from one genus to another (e.g. Cuvierimops: 1 lineage; Vespertiliavus: 6 lineages), and reacting more or less strongly to environmental changes associated with the Eocene–Oligocene transition. Moreover, the evolution of the dental characters and body sizes and the good documentation of the bat faunas over a long period of time (MP 13, ~44 Ma, to MP 25, ~29 Ma) significantly increased the temporal resolution of this evolutionary history and made it possible to use this group as a biochronological marker. Inferred body size increases and evolutionary trends over time (e.g., the proportional decrease in size of the premolars) in these lineages made it possible to precise the relative ages of most of the deposits studied, thus completing the biostratigraphic mammal scale of Europe. The order Chiroptera therefore appears to be, in much the same manner as the other mammals generally used for relative biochronology (such as rodents and ungulates), a good source of biochronological information, particularly in the palaeokarstic record. From this, the systematic and phyletic study of the bat fossil record from the late Oligocene and Neogne deposits of western Europe becomes possible, with the goal of obtaining a complete biochronological succession across all of the available data for an unrivaled period of time in the fossil record worldwide.
Quantitative analyses of body mass distributions (cenograms) have shown that the distribution of species’ body weights is closely related to the type of environment in which they live, even if fossil faunas appear to be biased in their larger species due to the absence of several large-sized families. This approach has helped to better understand some modalities of the evolution of this clade, notably with the preferential disappearance of taxa with extreme (small or large) body weight. The construction of bat cenograms for the reference levels of the upper Oligocene and Miocene, when the representatives of these large-sized families appeared, would likely help to better understand the evolution of the environment through that of the distribution of bat body weight classes.
References
Adaci, M., Tabuce, R., Mebrouk, F., Bensalah, M., Fabre, P.-H., Hautier, L., et al. (2007). Nouveaux sites à vertébrés paléogènes dans la région des Gour Lazib (Sahara nord-occidental, Algérie). Comptes Rendus Palevol, 6(8), 535–544.
Aguilar, J.-P. (2002). Les sciuridés des gisements karstiques du Miocène inférieur à moyen du sud de la France: nouvelles espèces, phylogénie, paléoenvironnement. Geobios, 35, 375–394.
Aguilar, J.-P. & Michaux, J. (1997). Les faunes karstiques néogènes du sud de la France et la question de leur homogénéité chronologique. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’97 (pp. 33–38). Mémoire des travaux de l’E.P.H.E. Institut de Montpellier.
Aguilar, J.-P., Michaux, J., Pélissié, T., & Sigé, B. (2007). Early late Pliocene paleokarstic fillings predating the major Plio-Pléistocene erosion of the Quercy table, SW-France. Acta Carsologica, 36(3), 469–473.
Allison, P. D. (2000). Multiple imputation for missing data: A cautionary tale. Sociological Methods and Research, 28, 301–309.
Astruc, J. G. (1988). Le paléokarst quercinois au Paléogène, altérations et sédimentations associées. Documents BRGM, 133.
Astruc, J.-G., Escarguel, G., Marandat, B., Simon-Coinçon, R., & Sigé, B. (2000). Floor-age constraining of a tectonic paroxysm of the Pyrenean orogen. Late Middle Eocene mammal age of a faulted karstic filling of the Quercy phosphorites, South-western France. Geodynamica Acta, 13, 271–280.
Astruc, J.-G., Hugueney, M., Escarguel, G., Legendre, S., Rage, J.-C., Simon-Coiçon, R., et al. (2003). Puycelci, nouveau site à vertébrés de la série molassique d’Aquitaine. Densité et continuité biochronologique de la zone Quercy et bassins périphériques au Paléogène. Geobios, 36, 629–648.
Augé, M. (2001). Les Lacertiliens (Reptilia, Squamata) du Paléogène d’Europe de l’Ouest: Paléobiodiversité, évolution, paléobiogéographie. Unpublished PhD Thesis, Muséum national d’Histoire naturelle de Paris.
(de) Bonis, L., Crochet, J.-Y., Rage, J.-C., Sigé, B., Sudre, J., & Vianey-Liaud, M. (1973). Nouvelles faunes de vertébrés oligocènes des Phosphorites du Quercy. Bulletin du Muséum National d’Histoire Naturelle, 3(174), 105–113.
Barghoorn, S. F. (1977). New material of Vespertiliavus Schlosser (Mammalia, Chiroptera) and suggested relationships of Emballonurid bats based on cranial morphology. American Museum Novitates, 2618, 1–29.
Billaud, Y. (1982). Les paragenèses phosphatées du paléokarst des Phosphorites du Quercy. Unpublished PhD Thesis, Université de Lyon1.
BiochroM’97. (1997). Synthèses et Tableaux de corrélations. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’97 (pp. 769–805). Mémoire des Travaux E.P.H.E, Institut Montpellier.
Brosset, A. (1966). La biologie des chiroptères. In Masson & Cie (Eds.), Les grands problèmes de la biologie. Paris.
CNRS, Mission biologique au Gabon. (1963–1969). Listes des vertébrés du bassin de L’Ivindo (République GABON).
Cirot, E. (1992). Etude phylogénétique de quelques genres d’Artoidea de l’Oligocène eurasiatique. Comparaison des données morphologiques et moléculaires. Unpublished PhD Thesis, Université de Poitiers.
Crochet, J.-Y. (1978). Les Marsupiaux du tertiaire d’Europe. Unpublished Habilitation Thesis, Université de Montpellier II.
Crochet, J.-Y., Hartenberger, J.-L., Rage, J.-C., Remy, J.-A., Sigé, B., Sudre, J., & Vianey- Liaud, M. (1981). Les nouvelles faunes de vertébrés antérieures à la “Grande Coupure” découvertes dans les Phosphorites du Quercy. Bulletin du Muséum National d’Histoire Naturelle, 4(3), 255–266.
Daubrée, A. (1871). Sur le gisement dans lequel la chaux phosphatée a été récemment découverte dans les départements de Tarn-et-Garonne et du Lot. Comptes rendus de l’Académie des Sciences de Paris, 43, 1029–1036.
de Franceschi, D., Le Gall, C., Escarguel, G., Hugueney, M., Legendre, S., Simon-Coinçon, R., et al. (2006). Une paléoflore des Phosphatières karstiques du Quercy (Sud-Ouest France): première découverte, résultats et perspectives. Strata, Ser., 1(13), 97–101.
Delfortrie, E. (1872). Les gites de chaux phosphat ée dans le déepartement du Lot; leur faune, le mode et l’ époque probable de leur formation. Actes de la Soci et e Linn eenne de Bordeaux, 28, 505–518.
Durand-Delga, M. (2006). De la découverte des phosphorites du Quercy au renouveau de leur étude avec Bernard Gèze. In Strata (Ed.), 30 millions d’années de biodiversité dynamique dans le paléokarst du Quercy (pp. 25–36). Lalbenque-Limogne.
Engesser, B. (1972). Die obermiozäne Säugetierfauna von Anwil (Baselland). Tätigkeitsberichte der naturforschenden Gesellschaft Baselland, 28, 1–363.
Escarguel, G. (1998). Les rongeurs de l’Eocène inférieur et moyen d’Europe: Systématique, Morphométrie, Evolution et Biochronologie des niveaux-repères MP 8-9 à MP 14. Unpublished PhD Thesis. Université Montpellier II.
Escarguel, G., & Legendre, S. (2006). New methods for analysing deep-time meta-community dynamics and their application to the Paleogene mammals from the Quercy and Limagne area (Massif Central, France). Strata, sér., 1(13), 245–273.
Escarguel, G., Legendre, S., & Sigé, B. (2008). Unearthing deep-time biodiversity changes: The Palaeogene mammalian metacommunity of the Quercy and Limagne area (Massif Central, France). Comptes rendus Geosciences, 340, 602–614.
Escarguel, G., Marandat, B. & Legendre, S. (1997). Sur l’âge numérique des faunes de mammifères du Paléogène d’Europe occidentale, en particulier celles de l’Eocène inférieur et moyen. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’97 (pp. 443–460). Mémoire des Travaux E.P.H.E., Institut de Montpellier.
Filhol, H. (1872). Recherches sur les mammifères fossiles des dépôts de phosphate de chaux dans les départements du Lot, du Tarn et de Tarn-et-Garonne. Annales des Sciences de Géologie, 3(7), 1–31.
Filhol, H. (1876). Recherche sur les Phosphorites du Quercy. Annales de la Société Géologique, 7, 44–48.
Furey, N. M., & Racey, P. A. (2007). Community composition of bat asssemblages in a Vietnamese karst protected area. Bat Research News, 48(3), 98–99.
Gervais, P. (1855). Zoologie. Mammifères. In F. de Castelnau (Ed.), Expédition dans les parties centrales de l’Amérique du Sud de Rio de Janeiro à Lima, et de Lima au Para: par ordre du gouvernement français pendant les années 1843 à 1847 (pp. 25–88).
Gèze, B. (1938). Contribution à la connaissance des Phosphorites du Quercy. Bulletin de la Société Géolologique de France, 5(VIII), 123–146.
Gèze, B., Rage, J.-C., Vergnaud-Grazzini, C., De Broin, F., Buffetaut, E., Mourer-Chauviré, C., Crochet, J.-Y., Sigé, B., Sudre, J., Remy, J.-A., Lange-Badré, B., De Bonis, L., Hartenberger, J.-L. & Vianey-Liaud, M. (1978). La poche à phosphate de Sainte-Néboule (Lot) et sa faune de vertébrés du Ludien supérieur. Palaeovertebrata, 8(2–4).
Godinot, M. (1983). Contribution à l’étude des primates paléogènes d’Europe: systématique, locomotion. Unpublished Habilitation Thesis, Université de Montpellier II.
Gradstein, J., Ogg, G., Schmitz, M., & Ogg, G. (2012). A Geologic Time Scale. Amsterdam: Elsevier.
Hand, S., Sigé, B. & Maitre, E. (2012). Necromantis Weithofer, 1887, large carnivorous Middle and late Eocene bats from the French Quercy Phosphorites: new data and unresolved relationships. In Gunnell, G.F. & Simmons, N. (eds.) Evolutionary history of bats: Fossils, molecules and morphology (pp. 210–251).
Happold, D.C.D. (1987). The mammals of Nigeria. In C. Press (Ed.), Oxford: Oxford Science publications (p. 402).
Hartenberger, J.-L. (1971). Les rongeurs de l’éocène d’Europe: leur évolution dans leur cadre biogéographique. Unpublished PhD Thesis, Université de Montpellier II, Montpellier.
Hartenberger, J.-L., Sigé, B., & Sudre, J. (1974). La plus ancienne faune de mammifères du Quercy: le Bretou. Palaeovertebrata, 6(3–4), 177–196.
Hooker, J. J., & Weidmann, M. (2000). The Eocene mammal faunas of Mormont. Schweitzerische Paläontologische Abhandlungen, 120, 1–133.
Horacek, I. (2001). On the early history of vespertilionid bats in Europe: the Lower Miocene record from the Bohemian Massif. Lynx, 32, 123–154.
Jones, K. E., Purvis, A., Maclarnon, A., Bininda-edmonds, O., & Simmons, N. (2002). A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biological Reviews, 77, 223–259.
Koopman, K. F. (1985). A synopsis of the families of bats, Part VII. Bat Research News, 25, 25–27.
Koopman, K. F. (1994). Chiroptera: Systematics. Handbook of zoology, Mammalia, 8, 1–217.
Lange-Badré, B. (1979). Les Créodontes (Mammalia) d’Europe occidentale de l’Eocène supérieur à l’Oligocène supérieur. Unpublished PhD Thesis, Muséum national d’histoire Naturelle de Paris.
Largen, M. J., Kock, D., & Yalden, D. W. (1974). Catalogue of the mammals of Ethiopia. Monitore Zoologica Italiano n. s., 16, 221–298.
Le Gall, C. (2001). Evaluation de la durée de dépôt d’un remplissage paléokarstique: Le cas de Baraval (Phosphorites du Quercy). Unpublished Master Thesis. Université Claude Bernard-Lyon 1.
Legendre, S. (1982). Etude anatomique de Tadarida helvetica (Chiroptera, Molossidae) du gisement burdigalien de Port-la-Nouvelle (Aude): denture et squelette appendiculaire. Zoologisches Jahrbuch, Abteilung für Anatomie, 108, 263–292.
Legendre, S. (1984a). Identification de deux sous-genres fossiles et compréhension phylogénique du genre Mormopterus (Molossidae, Chiroptera). Compte rendu de l’Académie des Sciences de Paris, 298(2, 16), 715–720.
Legendre, S. (1984b). Etude odontologique des représentants actuels du groupe Tadarida (Chiroptera, Molossidae). Implications phylogéniques, systématiques et zoogéographiques. Revue Suisse de Zoologie, 91(2), 399–442.
Legendre, S. (1985). Molossidés (Mammalia, Chiroptera) cénozoïques de l’Ancien et du Nouveau Monde; statut systématique; intégration phylogénique des données. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 170(2), 205–227.
Legendre, S. (1986). Analysis of mammalian communities from the late Eocene and Oligocene of southern France. Palaeovertebrata, 16, 191–212.
Legendre, S. (1987). Concordance entre paléontologie continentale et les événements paléocéanographiques: exemple des faunes de mammifères du Paléogène du Quercy. Compte rendu de l’Académie des Sciences de Paris, 304(3, 2), 45–49.
Legendre, S. (1988). Les communautés de mammifères du paléogène (éocène supérieur et oligocène) d’Europe occidentale: structures, milieux et évolution. Unpublishes Habilitation Thesis, Université de Montpellier II.
Legendre, S. (1989). Les communautés de mammifères du Paléogène (Eocène supérieur et Oligocène) d’Europe occidentale: structures, milieux et évolution. Münchner Geowissenschaftliche Abhandlungen (A), 16, 1–110.
Legendre, S., & Bachelet, B. (1993). The numerical ages: A new method of datation applied to Paleogene mammalian localities from Southern France. Newsletters on Stratigraphy, 29(3), 137–158.
Legendre, S. & Escarguel, G. (2006). Macroécologie an temps long: lignées et communautés de mammifères fossiles du Massif Central (Quercy et Limagnes, Eocène supérieur à Miocène inférieur). 21ème réunion des Sciences de la Terre, Symposium “Paléobiosphère” (Dijon, 4–8 décembre 2006, p. 197).
Legendre, S., & Hartenberger, J.-L. (1992). Evolution of Mammalian faunas in Europe during the Eocene and Oligocene. In D. Prothero (Ed.), Eocene–Oligocene climatic and biotic evolution (pp. 512–552). Princeton: Princeton University Press.
Legendre, S. & Sigé, B. (1982). La place du “Vespertilion de Montmartre” dans l’histoire des chiroptères molossidés. Actes du Symposium paléontologique Georges Cuvier, 347–361.
Legendre, S., Marandat, B., Remy, J.-A., Sigé, B., Sudre, J., Vianey-Liaud, M., et al. (1995). Coyrou 1-2, une nouvelle faune de mammifères des Phosphorites du Quercy, niveau intermédiaire (MP 20-21) proche de la “Grande Coupure”. Géologie de la France, 1, 63–68.
Legendre, S., Sigé, B., Astruc, J. G., de Bonis, L., Crochet, J.-Y., Denys, C., et al. (1997). Les phosphorites du Quercy: 30 ans de recherche. Bilan et perspectives. Geobios Mémoire spécial, 20, 331–345.
Legendre, S., Mourer-Chauviré, C., Hugueney, M., Maitre, E., Sigé, B., & Escarguel, G. (2006). Dynamique de la diversité des mammifères et des oiseaux paléogènes du Massif Central (Quercy et Limagnes, France). Strata, 13(1), 275–282.
Lydekker, RB. (1885). Catalogue of the fossil Mammalia in the British Museum. Part I. Primates, Chiroptera, Insectivora, Carnivora (p. 263). British Museum (Natural History), London.
Maitre, E. (2003). Etude paléontologique de la microfaune du gisement de Gardiol 3 (Phosphorites du Quercy, France) (pp. 1–20). Unpublished Master Thesis, Université Claude Bernard, Lyon1.
Maitre, E., Hugueney, M., Astruc, J.G., Crochet, J.-Y., Escarguel, G., Godinot, M., Legendre, S., Marandat, B., Mourer-Chauviré, C., Rage, J.-C., Remy, J.-A., Simon-Coinçon, R., Sudre, J., Valette, P., & Sigé, B. (2006a). Huit nouvelles faunes eocènes et oligocènes des Phosphorites du Quercy. Strata, 13(1), 113–127.
Maitre, E., Escarguel, G., & Sigé, B. (2006b). Amphilemuridae (Lipotyphla, Mammalia) éocènes d’Europe occidentale, nouvelles données taxonomiques. Comptes Rendus Palévol, 5(6), 813–820.
Maitre, E., Escarguel, G., & Sigé, B. (2008a). Amphilemuridae et autres Lipotyphla éocènes d’Europe occidentale, nouvelles données, systématique et phylogénie. Palaeontographica, 283, 35–82.
Maitre, E., Sigé, B., & Escarguel, G. (2008b). A new family of bats in the Paleogene of Europe: systematics and implications of emballonurids and rhinolophoids. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 250(2), 199–216.
Marandat, B., Crochet, J.-Y., Godinot, M., Hartenberger, J.-L., Legendre, S., Remy, J.-A., et al. (1993). Une nouvelle faune à mammifères d’âge éocène moyen (Lutétien supérieur) dans les Phosphorites du Quercy. Geobios, 26(5), 617–623.
Menu, H. (1987). Morphotypes dentaires actuels et fossiles des chiroptères Vespertilioninés. 2ème partie: implications systématiques et phylogéniques. Palaeovertebrata, 17(3), 77–150.
Menu, H. (1988). Sur le statut taxonomique de Myotis Kaup, 1829 (Mammalia, Chiroptera). Palaeovertebrata, 18(4), 263.
Menu, H., & Sigé, B. (1971). Nyctalodontie et myotodontie, importants caractères de grades évolutifs chez les chiroptères entomophages. Comptes rendus des séances de l’Académie des Sciences, 272, 1735–1738.
Miguet, R. (1967). Observations nouvelles sur les chiroptères des Phosphorites du Quercy. Travaux des Laboratoires de Géologie de la Faculté des Sciences de Lyon, 14, 103–114.
Miller, G. S. (1907). The families and genera of bats. Smithsonian institution, Bulletin of the United States National Museum, 57, 1–282.
Muratet, B., Crochet, J.-Y., Hartenberger, J.-L., Sigé, B., Sudre, J., & Vianey-Liaud, M. (1985). Nouveaux gisements à mammifères de l’Eocène supérieur et leur apport à la chronologie des épisodes sédimentaires et tectoniques de la bordure sud-ouest du Massif Central. Géologie de la France, 3, 271–286.
Perret, J. L., & Aellen, V. (1956). Mammifères du Cameroun de la collection J. L. Perret. Revue suisse de Zoologie, 63(26), 395–450.
Pictet, F.-J., Gaudin, C. & Harpe, D. L. (1855). Mémoires sur les animaux vertébrés du terrain sidérolithique du canton de Vaud. Matériaux pour la paléontologie suisse, 1(2), 120.
Pictet, F.-J. & Humbert, A. (1869). Mémoires sur les animaux vertébrés trouvés dans le terrain sidérolithique du canton de Vaud. Matériaux pour la paléontologie suisse supplément.
Rautenbach, I. L. (1982). Mammals of the Transvaal. Ecoplan Monograph, 1, 1–211.
Remy, J.-A., Aguilar, J.-P., Crochet, J.-Y., Duffaud, S., Escarguel, G., Godinot, M., Marandat, B., Michaux, J., Rage, J.-C., Sigé, B., Sudre, J. & Wiénin, M. (1997). Les remplissages karstiques polyphasés (Eocène, Oligocène, Pliocène) de Saint-Maximin (Phosphorites du Gard) et leur apport à la connaissance des faunes européennes, notamment pour l’Eocène moyen (MP 13). 1. Introduction, systématique (pars) et synthèse. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’97 (pp. 711–728). Mémoire des Travaux E.P.H.E., Institut de Montpellier, Montpellier.
Remy, J.-A., Crochet, J.-Y., Sigé, B., Sudre, J., De Bonis, L., Vianey-Liaud, M., et al. (1987). Biochronologie des phosphorites du Quercy: mise à jour des listes fauniques et nouveaux gisements de mammifères fossiles. Münchner Geowissenschaftliche Abhandlungen, 10, 169–188.
Revilliod, P. (1917a). Fledermaüse aus der Braunkhole von Messel bei Darmstadt. Abhandlungen der Grossherzoglich hessischen, 7(2), 159–196.
Revilliod, P. (1917, 1919, 1921–1922). Contribution à l’étude de chiroptères des terrains tertiaires. Mémoires de la Société Paléontologique suisse, 63–65.
Revilliod, P. (1919). L’état actuel de nos connaissances sur les chiroptères fossiles (note préléminaire). Extrait du compte rendu des séances de la société de physique et d’histoire naturelle de Genève, 38(3), 93–96.
Roger, O. (1896). Verzeichniss der bisher bek fossilen Säugetiere. Bericht naturwiss 32.
Russell, D. E., & Sigé, B. (1970). Révision des Chiroptères lutétiens de Messel (Hesse, Allemagne). Palaeovertebrata, 3(4), 83–182.
Schafer, J. L. (1999). NORM: Multiple imputation of incomplete multivariate data under a normal model, version 2. Software for Windows 95/98/NT. http://www.stat.psu.edu/~jls/misoftwa.html.
Schlosser, M. (1887). Die affen, Lemuren, Chiropteren, Insectivoren, Marsupialier, Creodonten und Carnivoren des europäischen Tertiärs und deren Beziehungen zu ihren lebenden und fossilen aussereuropäischen Verwandten. Beiträge zur paläontologie Österreich-Ungarns und des Orients, 6(1–2), 1–224.
Schmidt-Kittler, N. (ed.) (1987). International Symposium on Mammalian Biostratigraphy and Paleoecology of the European Paleogene. Münchner Geowissenschaftliche Abhandlungen, 10.
Sigé, B. (1966). Les chiroptères fossiles de Bouzigues (Hérault), recherches anatomiques sur Pseudorhinolophus bouziguensis n. sp. Unpublished PhD Thesis. Université Montpellier II, Montpellier.
Sigé, B. (1968). Les chiroptères du Miocène inférieur de Bouzigues. 1. Etude systématique. Palaeovertebrata, 1(3), 65–133.
Sigé, B. (1974a). Données nouvelles sur le genre Stehlinia (Vespertilionoidea, Chiroptera) du Paléogène d’Europe. Palaeovertebrata, 6, 253–272.
Sigé, B. (1974b). Insectivores et chiroptères de l’éocène supérieur et oligocène inférieur d’Europe occidentale. Article principal: insectivores primitifs de l’éocène supérieur et oligocène inférieur d’Europe occidentale. Nyctithériidés. Unpublished PhD Thesis, Université de Montpellier II.
Sigé, B. (1976). Les Megadermatidae (Chiroptera, Mammalia) miocènes de Béni Mellal, Maroc. Annale de l’Université de Provence, Géologie méditerranéenne, 3(2), 71–86.
Sigé, B. (1978). La poche à Phosphate de Ste-Néboule (Lot) et sa faune de vertébrés du Ludien supérieur. 8. Insectivores et chiroptères. Palaeovertebrata, 8(2–4), 243–268.
Sigé, B. (1985). Les chiroptères oligocènes du Fayum, Egypte. Geologica et Palaeontologica, 19, 161–189.
Sigé, B. (1988). Le gisement du Bretou (Phosphorites du Quercy, Tarn-et-Garonne, France) et sa faune de vertébrés de l’Eocène supérieur. IV. Insectivores et Chiroptères. Palaeontographica, 205, 69–102.
Sigé, B. (1990). Nouveaux chiroptères de l’Oligocène moyen des Phosphorites du Quercy, France. Compte rendu de l’Académie des Sciences de Paris, 310(2), 1131–1137.
Sigé, B. (1991). Rhinilophoidea et Vespertilionoidea (Chiroptera) du Chambi (Eocène inférieur de Tunisie). Aspects biostratigraphique, biogéographique et paléoécologique de l’origine des chiroptères modernes. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 182(3), 355–376.
Sigé, B. (1995). Le Garouillas et les sites contemporains (Oligocène, MP 25) des Phosphorites du Quercy (Lot, Tarn-et-Garonne, France) et leurs faune de vertébrés. 5. Chiroptères. Palaeontographica, 236, 77–124.
Sigé, B. (1997). Les remplissages karstiques polyphasés (Eocène, Oligocène, Pliocène) de St-Maximin (Phosphorites du Gard) et leur apport à la connaissance des faunes européennes, notamment pour l’Eocène moyen (MP 13). 3.- Systématique: Euthériens entomophages. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’97 (pp. 737–750). Mémoires des Travaux E.P.H.E., Institut Montpellier.
Sigé, B. & Crochet, J.-Y. (2006). Marsupiaux, insectivores s.l., chiroptères, créodontes et carnivores paléogènes d’Europe décrits ou révisés d’après les nouvelles collections du Quercy (SW France). In Strata (Ed.), 30 millions d’années de biodiversité dynamique dans le paléokarst du Quercy (pp. 189–205). Lalbenque-Limogne.
Sigé, B., Crochet, J.-Y., Hartenberger, J.-L., Rémy, J.-A., Sudre, J., & Vianey-Liaud, M. (1979). Mammifères du Quercy. In F. Westphal (Ed.), Fossilium Catalogus. I: Animalia. La Hague: Dr. W. Junk.
Sigé, B., Crochet, J.-Y., Sudre, J., Aguilar, J.-P., & Escarguel, G. (1997). Nouveaux sites d’âges variés dans les remplissages karstiques du Miocène inférieur de Bouzigues (Hérault, Sud de la France). Partie I: sites et faunes 1 (Insectivores, Chiroptères, Artiodactyles). Geobios Mémoire spécial, 20, 477–483.
Sigé, B. & Hugueney, M. (2006). Les micromammifères des gisements à Phosphate du Quercy (SW France). In Strata (Ed.), 30 millions d’années de biodiversité dynamique dans le paléokarst du Quercy (pp. 207–226). Lalbenque-Limogne.
Sigé, B., Hugueney, M., Crochet, J.-Y., Legendre, S., Mourer-Chauviré, C., Rage, J.-C., & Simon-Coiçon, R. (1998). Baraval, nouvelle faune de l’Oligocène inférieur (MP 22) des Phosphorites du Quercy. Apport à la signification chronologique des remplissages karstiques. Bulletin de la Société d’Histoire Naturelle de Toulouse, 134, 85–90.
Sigé, B., & Legendre, S. (1983). L’histoire des peuplements de chiroptères du Bassin méditerranéen: l’apport comparé des remplissages karstiques et des dépôts fluviolacustres. Mémoire Biospéoléologie, 10, 209–225.
Sigé, B. & Legendre, S. (1997). Un outil de la stratigraphie du Tertiaire continental: l’échelle de niveaux-repères de mammifères; spécificité; intérêt des faunes karstiques. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’ 97 (pp. 47–54). Mémoire et travaux de l’E.P.H.E., Institut de Montpellier.
Sigé, B., Maitre, E. & Hand, S. (2007). Necromantodonty, the primitive condition of lower molars among bats. In G. F. Gunnell & N. Simmons (Eds.), Evolutionary history of bats: Fossils, molecules and morphology (pp. 456–469).
Sigé, B., & Vianey-Liaud, M. (1979). Impropriété de la Grande Coupure de Stehlin comme support d’une limite Eocène–Oligocène. Newsletters on Stratigraphy, 8(1), 79–82.
Simpson, G. G. (1945). The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History, 85, 1–350.
Stehlin, H. G. (1909). Remarques sur les faunules de mammifères des couches éocènes et oligocènes du Bassin de Paris. Bulletin de la société géologique de France, 9(4), 488–620.
Storch, G., Sigé, B., & Habersetzer, J. (2002). Tachypteron franzeni n gen, nsp, earliest emballonurid bat from Middle Eocene of Messel (Mammaila, Chiroptera). Paläontologische Zeitschrift, 76(2), 189–199.
Sudre, J. (1977). Les artiodactyles de l’éocène moyen et supérieur d’Europe occidentale: systématique et évolution. Unpublished PhD Thesis, Université de Montpellier II, Montpellier.
Szalay, F. S. (1969). Mixodectidae, Microsyopidae, and the insectivore-primate transition. Bulletin of American Museum of Natural History, 140(4), 163–330.
Teilhard de Chardin, P. (1914–1915). Les carnassiers des Phosphorites du Quercy. Annales de Paléontologie, 9, 101–192.
Teilhard de Chardin, P. (1921). Sur quelques Primates des Phosphorites du Quercy. Annales de Paléontologie, 10, 1–20.
Thévenin, A. (1903). Etude géologique de la bordure Sud-Ouest du Massif Central. Bulletin du Service de la Carte géologique France, 14(95), 353–554.
Trouessart, E.-L. (1898–1899). Catalogus mammalium tam viventium quam fossilium. I., Primates, prosimiae, chiroptera, insectivora, carnivora, rodentia, pinnipedia. In Berolini, R. Friedländer und Sohn (Ed.), (p. 696). Berlin, Germany.
Trouessart, E.-L. (1904–1905). Catalogus mammalium tam viventium quam fossilium. I., Primates, prosimiae, chiroptera, insectivora, carnivora, rodentia, pinnipedia. In Berolini, R. Friedländer (Ed.), (pp. 289–546). Berlin, Germany.
Valverde, J. A. (1964). Remarques sur la structure et l’évolution des communautés de vertébrés terrestres. Revue d’Ecolologie (Terre et vie), 111, 121–154.
Valverde, J. A. (1967). Estructura de una communidad de vertebrados terrestres. Monographias de la Estacion biologica de Doñana, 1, 1–219.
Van Valen, L. M. (1966). Deltatheridia, a new order of mammals. Bulletin of the American Museum of Natural History, 132, 1–125.
Vianey-Liaud, M. (1971). Evolution des rongeurs à l’oligocène en Europe occidentale. Doctorat. Unpublished PhD Thesis, Université de Montpellier II.
Weithofer, A. (1887). Zur kenntniss der fossilen cheiropteren der französischens Phosphorite. Mathematisch-Naturwissenschaftlich Classe, 96, 341–360.
Quinet, G. E. (1965). Myotis misonnei n. sp. chiroptère de l’Oligocène de Hoogbutsel. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, 41(20), 1–11.
Yuan, Y. C. (2001). Multiple imputation for missing data: concepts and new development SAS/STAT 8.2. Cary: SAS Institute Inc (see http://www.sas.com/statistics)
Ziegler, R. (1993). Die chiroptera (Mammalia) aus dem Untermiozän von Wintershof-West bei Eichstätt (Bayern). Mitteilungen der Bayerischen Staatssammlung für Palälaontologie und Historische Geologie, 33, 119–154.
Ziegler, R. (2000). The bats (Chiroptera, Mammalia) from the Late Oligocene Fissure Fillings Herrlingen 8 and Herrlingen 9 near Ulm (Baden-Württemberg). Senckenbergiana Lethaea, 80(2), 647–684.
Zittel, K. (1891–1893). Handbuch der Paläontologie. I. Abth. Palaeozoologie IV Bd. Mammalia.
Acknowledgments
I thank Bernard Sigé and Gilles Escarguel (Université Claude Bernard Lyon 1) for supervising my PhD thesis that is the origin of this work. Suzanne Hand (University of New South Wales, Sydney) and Dominique Mouchiroud (Université Claude Bernard Lyon 1), Ivan Horacek (Charles University, Praha), Thierry Smith (Royal Belgian Institute of Natural Sciences, Bruxelles) and also Serge Legendre, Marguerite Hugueney, Cécile Mourer-Chauviré, and Pierre Mein (all four at the Université Claude Bernard Lyon 1) helped a lot through very constructive discussions either as members of my PhD committee for the four above-mentioned researchers or as members of the lab in Lyon. I thank the Earth Science department and the Laboratoire de Géologie of the University Claude Bernard Lyon 1 as well as the Society of Vertebrate Paleontology and the Conseil Général du Lot, France, for their financial help. I also thank all the persons in charge of the collections where the material is stored: Monique Vianey-Liaud and Bernard Marandat (Université Montpellier 2, France), Arne Ziems and Burkart Engesser (Naturhistorisches Museum Basel, Switzerland), Edmée Ladier (Muséum d’Histoire Naturelle de Montauban, France), and Abel Prieur (Centre de Conservation et de Collections de Géologie de l’Université de Lyon, France). Finally, I thank Loïc Costeur (Naturhistorisches Museum Basel, Switzerland) for editing this long manuscript and making its publication possible. I thank Daniel Marty for his extensive editorial work and Susanne Hand and Gilles Escarguel for thorough reviews that greatly improved the quality of the paper. Translation of the text into English was made possible through financial help of the Geoscience Department (Geowissenschaften Abteilung) of the Naturhistorisches Museum Basel.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maitre, E. Western European middle Eocene to early Oligocene Chiroptera: systematics, phylogeny and palaeoecology based on new material from the Quercy (France). Swiss J Palaeontol 133, 141–242 (2014). https://doi.org/10.1007/s13358-014-0069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13358-014-0069-3