Abstract
The purpose of this study was to develop and evaluate nanoemulsions (NEs) containing besifloxacin for ocular drug delivery. Pseudo ternary phase diagrams were constructed using Triacetin (oil), Cremophor® RH 40 (surfactant), and Transcutol®P (co-surfactant) to identify NE regions. Six formulations were developed by low-energy emulsification method and then evaluated for size, refractive index, pH, osmolality, viscosity, and drug release. After accelerated physical stability and bovine conrneal permeation studies, NE2 was chosen as optimized formulation forantimicrobial efficacy, and hen’s egg test-chorioallantoic membrane (HET-CAM) tests. The particle size of optimum NE was 14 nm with a narrow size distribution. Moreover, other physicochemical characterizations were in the acceptable range for ocular administration. Besifloxacin-loaded NEs demonstrated sustained release pattern and 1.7-fold higher permeation compared with the control suspension in the ex vivo transcorneal permeation study. HET-CAM test indicated no irritation, and HL% revealed no damage to the tissue, so the optimum NE is well tolerated by the eye. In vitro antimicrobial evaluation, showed comparative efficacy of lower drug-loaded NE (0.2%) versus 0.6% besifloxacin suspension (equal concentration to commercial besifloxacin eye drop). In conclusion, besifloxacin-loaded NEs could be considered as a suitable alternative to the marketed suspension for treating bacterial eyeinfections.
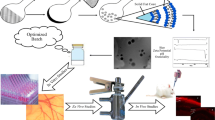
Graphical abstract







Similar content being viewed by others
References
Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017. https://doi.org/10.1186/s12886-017-0612-2.
O’Callaghan RJ. The Pathogenesis of Staphylococcus auerus Eye Infections. Pathogens. 2018. https://doi.org/10.3390/pathogens7010009.
Mahvan TD, Hornecker JR, Buckley WA, Clark S. The role of besifloxacin in the treatment of bacterial conjunctivitis. Ann Pharmacother. 2014. https://doi.org/10.1177/1060028014524175
Rahimi F, Hashemian MN, Khosravi A, Moradi G, Bamdad S. Bacterial keratitis in a tertiary eye centre in Iran: a retrospective study. Middle East Afr J Ophthalmol. 2015. https://doi.org/10.4103/0974-9233.151870.
Tótoli EG, Salgado HRN. Besifloxacin: a critical review of its characteristics, properties, and analytical methods. Crit Rev Anal Chem. 2018. https://doi.org/10.1080/10408347.2018.1429885.
Proksch JW, Granvil CP, Siou-Mermet R, Comstock TL, Paterno MR, Ward KW. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J Ocul Pharmacol Ther. 2009. https://doi.org/10.1089/jop.2008.0116.
Miller D, Chang JS, Flynn HW, Alfonso EC. Comparative in vitro susceptibility of besifloxacin and seven comparators against ciprofloxacin- andmethicillin-susceptible/nonsusceptible staphylococci. J Ocul Pharmacol Ther. 2013. https://doi.org/10.1089/jop.2012.0081
Haas W, Pillar CM, Zurenko GE, Lee JC, Brunner LS, Morris TW. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009. https://doi.org/10.1128/AAC.00418-09.
DeLeon J, Silverstein BE, Allaire C, Gearinger LS, Bateman KM, Morris TW, Comstock TL. Besifloxacin ophthalmic suspension 0.6% administered twice daily fo 3 days in the treatment of bacterial conjunctivitis in adults and children. Clin Drug Investig. 2012. https://doi.org/10.2165/11632470-000000000-00000
Morrison PW, Khutoryanskiy VV. Advances in ophthalmic drug delivery. Ther Deliv. 2014. https://doi.org/10.4155/tde.14.75.
McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012. https://doi.org/10.1039/C2SM06903B.
Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016. https://doi.org/10.1039/c5sm02958a.
Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002. https://doi.org/10.1016/s1350-9462(01)00017-9.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potentialophthalmic delivery system for dorzolamide hydrochloride. AAPS Pharm Sci Tech. 2009a. https://doi.org/10.1208/s12249-009-9268-4.
Khan KA, Rhodes CT. Effect of compaction pressure onthe dissolution efficiency of some direct compression systems. Pharm Acta Helv. 1972;47:594–607.
Djordjevic L, Primorac M, Stupar M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm. 2005. https://doi.org/10.1016/j.ijpharm.2005.02.014.
Chen H, Mou D, Du D, Chang X, Zhu D, Liu J, Xu H, Yang X. Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm. 2007. https://doi.org/10.1016/j.ijpharm.2007.03.052.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007. https://doi.org/10.1016/j.ejpb.2006.10.014.
Butani D, Yewale C, Misra A. Amphotericin B topical microemulsion:formulation, characterization and evaluation. Colloids Surf B Biointerfaces. 2014. https://doi.org/10.1016/j.colsurfb.2014.01.014.
Liu Z, Zhang X, Wu H, Li J, Shu L, Liu R, Li L, Li N. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev Ind Pharm. 2011. https://doi.org/10.3109/03639045.2010.522193
Alany RG, Rades T, Nicoll J, Tucker IG, Davies NM. W/O microemulsions for ocular delivery: evaluation of ocular irritation and precorneal retention. J Control Release. 2006. https://doi.org/10.1016/j.jconrel.2005.11.020.
Shah J, Nair AB, Jacob S, Patel RK, Shah H, Shehata TM, Morsy MA. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics. 2019. https://doi.org/10.3390/pharmaceutics11050230.
Pathak MK, Chhabra G, Pathak K. Design and development of a novel pH triggered nanoemulsified in-situ ophthalmic gel of fluconazole: ex-vivo transcorneal permeation, corneal toxicity and irritation testing. Drug Dev Ind Pharm. 2013. https://doi.org/10.3109/03639045.2012.707203.
Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 2004. https://doi.org/10.1016/j.ejpb.2004.03.033.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS Pharm Sci Tech. 2009b. https://doi.org/10.1208/s12249-009-9268-4.
Patel N, Nakrani H, Raval M, Sheth N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016. https://doi.org/10.1080/10717544.2016.1223225.
Mahboobian MM, Seyfoddin A, Aboofazeli R, Foroutan SM, Rupenthal ID. Brinzolamide-loaded nanoemulsions: ex vivo transcorneal permeation, cell viability and ocular irritation tests. Pharm Dev Technol. 2019. https://doi.org/10.1080/10837450.2018.1547748.
Fouad SA, Basalious EB, El-Nabarawi MA, Tayel SA. Microemulsion and poloxamer microemulsion-based gel for sustained transdermal delivery of diclofenac epolamine using in-skin drug depot: in vitro/in vivo evaluation. Int J Pharm. 2013. https://doi.org/10.1016/j.ijpharm.2013.06.009.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Dev Ind Pharm. 2010. https://doi.org/10.3109/03639041003801885.
Tayel SA, El-Nabarawi MA, Tadros MI, Abd-Elsalam WH. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int J Pharm. 2013. https://doi.org/10.1016/j.ijpharm.2012.12.049.
Raval N, Khunt D, Misra M. Microemulsion-based delivery of triamcinolone acetonide to posterior segment of eye using chitosan and butter oil as permeation enhancer: an in vitro and in vivo investigation. J Microencapsul. 2018. https://doi.org/10.1080/02652048.2018.1425750.
United State Pharmacopeia, Ophthalmic Products-Quality Tests, in USP39-NF34, 2016. pp. 589–595.
Fialho SL, da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Exp Ophthalmol. 2004;32:626–32.
Kesavan K, Pandit JK, Kant S, Muthu MS. Positively charged microemulsions of dexamethasone: comparative effects of two cosurfactants on ocular drug delivery and bioavailability. Ther Deliv. 2013. https://doi.org/10.4155/tde.13.106.
Zignani M, Tabatabay C, Gurny R. Topical semi-solid drug delivery: kinetics and tolerance of ophthalmic hydrogels. Adv Drug Deliv Rev. 1995. https://doi.org/10.1016/0169-409X(95)00015-Y.
Radomska-Soukharev A, Wojciechowska J. Microemulsions as potential ocular drug delivery systems: phase diagrams and physical properties depending on ingredients. Acta Pol Pharm. 2005;62:465–71.
Pathak K, Pattnaik S, Swain K. Application of nanoemulsions in drug delivery. in: Jafari SM, McClements DJ editors. Nanoemulsions, Elsevier Inc; 2018;415–433
Peters MCC, Santos Neto ED, Monteiro LM, Yukuyama MN, Machado MGM, de Oliveira IF, Zanin MHA, Löbenberg R, Bou-Chacra N. Advances in ophthalmic preparation: the role of drug nanocrystals and lipid-based nanosystems. J Drug Target. 2019. https://doi.org/10.1080/1061186X.2019.1663858.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, Chourasia MK. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017. https://doi.org/10.1016/j.jconrel.2017.03.008.
Mahboobian MM, Seyfoddin A, Rupenthal ID, Aboofazeli R, Foroutan SM. Formulation development and evaluation of the therapeutic efficacy of brinzolamide containing nanoemulsions. Iran J Pharm Res. 2017;16:847–57.
Soliman OAE, Mohamed EA, Khatera NAA. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: formulation, optimization, and in vitro-in vivo evaluation. Pharm Dev Technol. 2019. https://doi.org/10.1080/10837450.2017.1413658.
Kumar R, Sinha VR. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf B Biointerfaces. 2014. https://doi.org/10.1016/j.colsurfb.2014.02.007.
Calvo P, Vila-Jato JL, Alonso MJ. Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J Pharm Sci. 1996. https://doi.org/10.1021/js950474+.
Muchtar S, Abdulrazik M, Frucht-Pery J, Benita S. Ex-vivo permeation study of indomethacin from a submicron emulsion through albino rabbit cornea. J Control Release. 1997. https://doi.org/10.1016/S0168-3659(96)01503-9.
Schoenwald RD, Huang HS. Corneal penetration behavior of beta-blocking agents I: physiochemical factors. J Pharm Sci. 1983. https://doi.org/10.1002/jps.2600721108.
Dholakiya SL, Barile FA. Alternative methods for ocular toxicology testing: validation, applications and troubleshooting. Expert Opin Drug Metab Toxicol. 2013. https://doi.org/10.1517/17425255.2013.783013.
Wilson SL, Ahearne M, Hopkinson A. An overview of current techniques for ocular toxicity testing. Toxicology. 2015. https://doi.org/10.1016/j.tox.2014.11.003.
Üstündag-Okur N, Gökçe EH, Eğrilmez S, Özer Ö, Ertan G. Novel ofloxacin-loaded microemulsion formulations for ocular delivery. J Ocul Pharmacol Ther. 2014. https://doi.org/10.1089/jop.2013.0114.
Funding
This research was financially supported (Grant No. 9704192161) by the Hamadan University of Medical Sciences, Hamadan, I.R. Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kassaee, S.N., Mahboobian, M.M. Besifloxacin-loaded ocular nanoemulsions: design, formulation and efficacy evaluation. Drug Deliv. and Transl. Res. 12, 229–239 (2022). https://doi.org/10.1007/s13346-021-00902-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00902-z