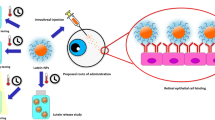
Abstract
Lutein has various biological activities, its application in food and pharma industries are limited due to poor aqueous solubility, stability, and bioavailability. To achieve various benefits, lutein-poly (lactic-co-glycolic acid) (PLGA)-phospholipid (PL) nanocapsules were prepared. Lutein-PLGA NCs (+PL) were synthesized, characterized and its bioavailability was studied in vitro and in vivo. The cellular uptake and anti-proliferative activity were analyzed in Hep G2 cells. The mean size and zeta value of lutein-PLGA NCs (+PL) were 140 ± 6 nm and − 44 mV. The amorphous nature of lutein in PLGA NCs (+PL) was confirmed by XRD and DSC. In vitro lutein release kinetics showed an initial burst followed by sustainable release up to 86%. In vitro bioavailability showed 62.7% higher lutein bioaccessibility than lutein in free form. The AUC of lutein after single oral dose of lutein-PLGA NCs (+PL) revealed 3.91-fold (plasma), 2.89-fold (liver), and 3.12-fold (eyes) higher absorption than the control (mixed micelles). The IC50 of lutein-PLGA NCs (+PL) in Hep G2 cells at 72 h was 4.5 μM as opposed to 23.4 μM for lutein in free form. Thus, results reveal that PL added to PLGA NCs helps in enhancing the solubility which in turn resulted in its better bioavailability and bioefficacy.









Similar content being viewed by others
References
Wang M, Tsao R, Zhang S, Dong Z, Yang R, Gong J, et al. Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anti-clastogenicity of lutein from marigold flowers. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2006;44:1522–9.
Chew BP, Wong MW, Wong TS. Effects of lutein from marigold extract on immunity and growth of mammary tumors in mice. Anticancer Res. 1996;16:3689–94.
Wu W, Li Y, Wu Y, Zhang Y, Wang Z, Liu X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res. 2015;59:1663–73.
Xu X-R, Zou Z-Y, Xiao X, Huang Y-M, Wang X, Lin X-M. Effects of lutein supplement on serum inflammatory cytokines, ApoE and lipid profiles in early atherosclerosis population. J Atheroscler Thromb. 2013;20:170–7.
Koushan K, Rusovici R, Li W, Ferguson LR, Chalam KV. The role of lutein in eye-related disease. Nutrients. 2013;5:1823–39.
Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201.
Lienau A, Glaser T, Tang G, Dolnikowski GG, Grusak MA, Albert K. Bioavailability of lutein in humans from intrinsically labeled vegetables determined by LC-APCI-MS. J Nutr Biochem. 2003;14:663–70.
Yoo JH, Shanmugam S, Thapa P, Lee E-S, Balakrishnan P, Baskaran R, et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch Pharm Res. 2010;33:417–26.
Kotake-Nara E, Nagao A. Absorption and metabolism of xanthophylls. Mar Drugs. 2011;9:1024–37.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B: Biointerfaces. 2010;75:1–18.
Arunkumar R, Harish Prashanth KV, Baskaran V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013;141:327–37.
Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr. 2001;131:2921–7.
Mamatha BS, Baskaran V. Effect of micellar lipids, dietary fiber and β-carotene on lutein bioavailability in aged rats with lutein deficiency. Nutr Burbank Los Angel Cty Calif. 2011;27:960–6.
Arunkumar R, Prashanth KVH, Manabe Y, Hirata T, Sugawara T, Dharmesh SM, et al. Biodegradable poly (lactic-co-glycolic acid)–polyethylene glycol nanocapsules: an efficient carrier for improved solubility, bioavailability, and anticancer property of lutein. J Pharm Sci. 2015;104:2085–93.
Garrett DA, Failla ML, Sarama RJ. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J Agric Food Chem. 1999;47:4301–9.
Report of the American Institute of Nurtition ad hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–8.
Baskaran V, Sugawara T, Nagao A. Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids. 2003;38:705–11.
Serpeloni JM, Barcelos GR, Friedmann JA, Mercadante AZ, Lourdes MPB, Antunes LM. Dietary carotenoid lutein protects against DNA damage and alterations of the redox status induced by cisplatin in human derived HepG2 cells. Toxicol Vitro Int J Publ Assoc BIBRA. 2012;26:288–94.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–75.
Shanmugam S, Park J-H, Kim KS, Piao ZZ, Yong CS, Choi H-G, et al. Enhanced bioavailability and retinal accumulation of lutein from self-emulsifying phospholipid suspension (SEPS). Int J Pharm. 2011;412:99–105.
Feng S-S. Nanoparticles of biodegradable polymers for new-concept chemotherapy. Expert Rev Med Devices. 2004;1:115–25.
Niamprem P, Rujivipat S, Tiyaboonchai W. Development and characterization of lutein-loaded SNEDDS for enhanced absorption in Caco-2 cells. Pharm Dev Technol. 2014;19:735–42.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86:1–12.
Shi X-M, Chen F. Stability of lutein under various storage conditions. Food Nahrung. 1997;41:38–41.
Anarjan N, Tan CP. Effects of storage temperature, atmosphere and light on chemical stability of astaxanthin nanodispersions. J Am Oil Chem Soc. 2013;90:1223–7.
Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int J Pharm. 2011;414:267–75.
Shanmugam S, Baskaran R, Balakrishnan P, Thapa P, Yong CS, Yoo BK. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Für Pharm Verfahrenstechnik EV. 2011;79:250–7.
Felice B, Prabhakaran MP, Rodríguez AP, Ramakrishna S. Drug delivery vehicles on a nano-engineering perspective. Mater Sci Eng C. 2014;41:178–95.
Joshi N, Saha R, Shanmugam T, Balakrishnan B, More P, Banerjee R. Carboxymethyl-chitosan-tethered lipid vesicles: hybrid nanoblanket for oral delivery of paclitaxel. Biomacromolecules. 2013;14:2272–82.
Deming DM, Erdman JW. Mammalian carotenoid absorption and metabolism. Pure Appl Chem [Internet]. 1999 [cited 2016 Jan 21];71. Available from: http://www.degruyter.com/view/j/pac.1999.71.issue-12/pac199971122213/pac199971122213.xml
Lakshminarayana R, Raju M, Keshava Prakash MN, Baskaran V. Phospholipid, oleic acid micelles and dietary olive oil influence the lutein absorption and activity of antioxidant enzymes in rats. Lipids. 2009;44:799–806.
Vishwanathan R, Wilson TA, Nicolosi RJ. Bioavailability of a nanoemulsion of lutein is greater than a lutein supplement. Nano Biomed Eng. 2009;1:38–49.
des Rieux A, Ragnarsson EG, Gullberg E, Préat V, Schneider Y-J, Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur J Pharm Sci. 2005;25:455–65.
Acknowledgments
R. Arunkumar acknowledges Indian Council of Medical Research (ICMR), Govt. of India for awarding Senior Research Fellowship.
Funding
This research project was financially supported by the Department of Science and Technology (DST), Govt. of India (DST, INT/JSPS/P-122/11)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
Manuscripts submitted for publication must contain a declaration that the experiments comply with the current laws of the country in which they were performed.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1835 kb)
Rights and permissions
About this article
Cite this article
Ranganathan, A., Manabe, Y., Sugawara, T. et al. Poly (d, l-lactide-co-glycolide)-phospholipid nanocarrier for efficient delivery of macular pigment lutein: absorption pharmacokinetics in mice and antiproliferative effect in Hep G2 cells. Drug Deliv. and Transl. Res. 9, 178–191 (2019). https://doi.org/10.1007/s13346-018-0590-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0590-9