Abstract
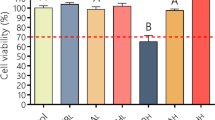
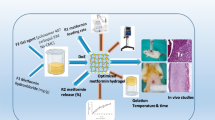
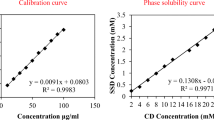
The purpose of this work was to develop an effective carbomer hydrogel to be used to treat second-degree burns that combined ciprofloxacin and lidocaine (CbCipLid hydrogel). Its antibiotic and anesthetic efficacy and the physical and chemical properties of the CbCipLid hydrogel (release rate and kinetics, rheology, appearance, and drug content) were evaluated both before and after a sterilization cycle and also after 6 months of storage. For the in vivo studies, second-degree burns were developed in a rat model. Animals were divided into three groups: CbCipLid hydrogel, silver sulfadiazine cream (reference), and carbomer hydrogel (as control). The treatments were applied daily for 21 days, and the healing was monitored by macroscopic observation and histologic evaluation. The anesthetic effect was evaluated through the corneal touch threshold in a rabbit eye model. The CbCipLid hydrogel obtained is transparent and allows the loading of ciprofloxacin above its solubility at a neutral pH, with a rheology which is convenient for topical administration. Its physical and chemical properties remained unchanged after sterilization and for at least six additional months. Both ciprofloxacin and lidocaine are reversibly released from the CbCipLid hydrogel with a kinetics fitting the Higuchi model. The presence of a biologic-like fluid increased the rate of drug delivery through an ionic exchange mechanism. Treatment with the CbCipLid hydrogel decreased the wound-healing period, compared with the reference, and was associated with a greater number of fibroblasts and a faster rate of epithelialization and dermis reconstruction. These differences were assigned to the moist environment provided by the hydrogel and also to the presence of a therapeutic concentration of ciprofloxacin. Moreover, CbCipLid hydrogel provides an immediate anesthetic effect, which is significantly more intense than that of the reference. Based on these results, it is believed that the CbCipLid hydrogel could be a potential candidate in the prophylaxis/treatment of second-degree burns.









Similar content being viewed by others
References
World Health Organization. Burns [Internet]. Fact sheet No. 365. 2017. Available from: http://www.who.int/mediacentre/factsheets/fs365/en/
Li J, Zhang Y-P, Zarei M, Zhu L, Sierra JO, Mertz PM, et al. A topical aqueous oxygen emulsion stimulates granulation tissue formation in a porcine second-degree burn wound. Burns [Internet]. Elsevier Ltd and International Society of Burns Injuries. 2015;41(5):1049–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25554261
Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane database Syst Rev. England; 2013;(3):CD002106.
Alemdaroǧlu C, Deǧim Z, Çelebi N, Zor F, Öztürk S, Erdoǧan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32(3):319–27.
Roy DC, Tomblyn S, Isaac KM, Kowalczewski CJ, Burmeister DM, Burnett LR, et al. Ciprofloxacin-loaded keratin hydrogels reduce infection and support healing in a porcine partial-thickness thermal burn. Wound Repair Regen [Internet]. 2016;24(4):657–68. https://doi.org/10.1111/wrr.12449.
ANMAT. Vademécum Nacional de Medicamentos: Platsul A [Internet]. 2018. Available from: http://anmatvademecum.servicios.pami.org.ar/index.html. Accessed 12 Feb 2018
Yaman I, Durmus a S, Ceribasi S, Yaman M. Effects of Nigella sativa and silver sulfadiazine on burn wound healing in rats. Vet Med (Praha) 2010. 55(12):619–24.
Tarameshloo M, Norouzian M, Zarein-Dolab S, Dadpay M, Gazor R. A comparative study of the effects of topical application of Aloe vera, thyroid hormone and silver sulfadiazine on skin wounds in Wistar rats. Lab Anim Res [Internet]. 2012;28(1):17–21. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3315193&tool=pmcentrez&rendertype=abstract
Hoeksema H, Vandekerckhove D, Verbelen J, Heyneman A, Monstrey S. A comparative study of 1% silver sulphadiazine (Flammazine) versus an enzyme alginogel (Flaminal) in the treatment of partial thickness burns. Burns [Internet]. Elsevier Ltd Int Soc Burns Injuries. 2013;39(6):1234–41. https://doi.org/10.1016/j.burns.2012.12.019.
Bagri LP, Bajpai J, Bajpai AK. Evaluation of starch based cryogels as potential biomaterials for controlled release of antibiotic drugs. Bull Mater Sci. 2011;34(7):1739–48.
Abdelkader H, Mansour HF. Comparative studies for ciprofloxacin hydrochloride pre-formed gels and thermally triggered (in situ) gels: in vitro and in vivo appraisal using a bacterial keratitis model in rabbits. Pharm Dev Technol England. 2015;20(4):410–6.
Olivera ME, Manzo RH, Junginger HE, Midha KK, Shah VP, Stavchansky S, et al. Biowaiver monographs for immediate release solid oral dosage forms: ciprofloxacin hydrochloride. J Pharm Sci United States. 2011;100(1):22–33.
Cassens J, Prudic A, Ruether F, Sadowski G. Solubility of pharmaceuticals and their salts as a function of pH. Ind Eng Chem Res. 2013;2731(1):2721–31.
Lubrizol. Carbopol® Polymer Products [Internet]. 2018. Available from: https://www.lubrizol.com/Life-Sciences/Products/Carbopol-Polymer-Products
Vilches AP, Jimenez-Kairuz A, Alovero F, Olivera ME, Allemandi DA, Manzo RH. Release kinetics and up-take studies of model fluoroquinolones from carbomer hydrogels. Int J Pharm. 2002;246(1–2):17–24.
Bermúdez JM, Jimenez-Kairuz AF, Olivera ME, Allemandi DA, Manzo RH. A ciprofloxacin extended release tablet based on Swellable drug polyelectrolyte matrices. AAPS PharmSciTech [Internet]. 2008;9(3):924–30. Available from:. https://doi.org/10.1208/s12249-008-9098-9.
Ministerio de Salud; Secretaría de Políticas Regulación e Institutos; Administración Nacional de Medicamentos Alimentos y Tecnología Médica; Instituto Nacional de Medicamentos. Farmacopea Argentina [Internet]. 7th ed. Comisión Permanente de la Farmacopea Argentina, editor. Ciudad Autónoma de Buenos Aires; 2003. Available from: http://www.anmat.gov.ar/webanmat/fna/fna.asp
ANMAT. Vademecum Nacional de Medicamentos: fotamicin [Internet]. 2018. Available from: http://anmatvademecum.servicios.pami.org.ar/index.html. Accessed 12 Feb 2018
Dash S, Murthy PN, Nath L, Chowdhary P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
The United States Pharmacopeial Convention. The United States Pharmacopeia and The National Formulary. The United States Pharmacopeia 40 The National Formulary 35 [Internet]. USP40 NF35. United States Pharmacopoeial Convention, Washington, DC; 2017. Accessed 12 Feb 2018. Available from: http://www.uspnf.com/uspnf/pub/index?usp=37&nf=32&s=2&officialOn=December 1, 2014.
International Conference on Harmonization (ICH). ICH topic Q2 (R1) validation of analytical procedures: text and methodology. Int Conf Harmon. 2005;4:1–13.
Gomes MT, Campos GRS, Piccolo N, França CM, Guedes GH, Lopes F, et al. Experimental burns: comparison between silver sulfadiazine and photobiomodulation. Rev Assoc Med Bra. 2017;63(1):29–34.
Turatti Pessolato AG, dos SantosMartins D, Ambrósio CE, Mananares CAF, de Carvalho AF. Propolis and amnion reepithelialise second-degree burns in rats. Burns. 2011;37(7):1192–201.
Han M, Durmus A, Karabulut E, Yaman I. Effects of Turkish Propolis and silver sulfadiazine on burn wound healing in rats. Rev Méd Vét. 2005;156(12):624–7.
McAlvin JB, Zhan C, Dohlman JC, Kolovou PE, Salvador-Culla B, Kohane DS. Corneal anesthesia with site 1 sodium channel blockers and dexmedetomidine. Investig Ophthalmol Vis Sci. 2015;56(6):3820–6.
Sharrow-Reabe KL, Townsend WM. Effects of action of proparacaine and tetracaine topical ophthalmic formulations on corneal sensitivity in horses. J Am Vet Med Assoc [Internet] 2012;241(12):1645–1649. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L366342142%5Cnhttp://avmajournals.avma.org/doi/pdf/https://doi.org/10.2460/javma.241.12.1645%5Cnhttp://dx.doi.org/10.2460/javma.241.12.1645%5Cnhttp://novacat.nova.edu:4550/resserv?sid=EMBASE&issn=00031488.
Wang L, Shankarappa S, Tong R, Ciolino J, Tsui J, Chiang H, et al. Topical drugs formulations for prolonged corneal anesthesia. Cornea. 2013;32(7):1040–5.
Golberg A, Khan S, Belov V, Quinn KP, Albadawi H, Felix Broelsch G, et al. Skin rejuvenation with non-invasive pulsed electric fields. Sci Rep [Internet]. 2015;5:10187. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4428072&tool=pmcentrez&rendertype=abstract
Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci [Internet]. 2015;104(11):3653–80. https://doi.org/10.1002/jps.24610.
Sinha M, Banik RM, Haldar C, Maiti P. Development of ciprofloxacin hydrochloride loaded poly(ethylene glycol)/chitosan scaffold as wound dressing. J Porous Mater. 2013;20(4):799–807.
World Health Organization (WHO). Antimicrobial resistance. Global report on surveillance [Internet]. Switzerland. World Health Organization (WHO). 2014. 232 p. Available from: http://www.who.int/drugresistance/en/%0AISBN
Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130(3):202–15.
Dua K, Malipeddi VR, Madan J, Gupta G, Jesus TDE, Pinto A. Norfloxacin and metronidazole topical formulations for effective treatment of bacterial infections and burn wounds. Interv Med Appl Sci. 2016;8(2):68–76.
Jacobsen F, Fisahn C, Sorkin M, Thiele I, Hirsch T, Stricker I, et al. Efficacy of topically delivered moxifloxacin against wound infection by Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(5):2325–34.
Jimenez-kairuz A, Allemandi D, Manzo RH. Mechanism of lidocaine release from carbomer ± lidocaine hydrogels. J Pharm Sci. 2002;91(1):267–72.
Esteban SL, Manzo RH, Alovero FL. Azithromycin loaded on hydrogels of carbomer: chemical stability and delivery properties. Int J Pharm. 2009;366:53–7.
Guzmán ML, Soria EA, Laino C, Manzo RH, Olivera ME. Reduced food interaction and enhanced gastrointestinal tolerability of a new system based on risedronate complexed with eudragit e100. Mechanistic approaches from in vitro and in vivo studies. Eur J Pharm Biopharm. 2016;107:263–72.
Battistini FD, Boiero C, Palma SD, Allemandi DA, Manzo RH, Olivera ME, et al. The role of hyaluronan as a drug carrier to enhance the bioavailability of extended release ophthalmic formulations. Hyaluronan-timolol ionic complexes as a model case. Eur J Pharm Sci. 2017;49(4):588–94.
Alovero FL, Olivera ME, Manzo RH. In vitro pharmacodynamic properties of a fluoroquinolone pharmaceutical derivative: hydrochloride of ciprofloxacin-aluminium complex. Int J Antimicrob Agents. 2003;21(5):446–51.
Clinical and Laboratory Standars Institute (CLSI). EM100 Connect-CLSI M100 S27:2017 [Internet]. 2017. Available from: http://em100.edaptivedocs.info/GetDoc.aspx?doc=CLSI M100 S27:2017&scope=user.
Quinteros DA, Rigo VR, Kairuz AFJ, Olivera ME, Manzo RH, Allemandi DA. Interaction between a cationic polymethacrylate (Eudragit E100) and anionic drugs. Eur J Pharm Sci Netherlands. 2008;33(1):72–9.
Barry BW, Meyer MC. Rheological properties of carbopol gels: 1. Continuous shear and creep properties of carbopol gels. Int J Pharm. 1979;2:1–25.
Pena LE. Gel dosage forms: theory, formulation and processing. In: Oborne D, Amann A, editors. Drug and the pharmaceutical sciences. New York: Marcel Dekker; 1990. p. 381–8.
Bousmina M. Rheology of polymer blends: linear model for viscoelastic emulsions. Rheol Acta. 1999;38(1):73–83.
Ruiz Martinez MA, Lopez-Viota Gallardo J, de Benavides MM, de Dios Garcia Lopez-Duran J, Gallardo Lara V. Rheological behavior of gels and meloxicam release. Int J Pharm. 2007;333(1–2):17–23.
Kutschmann E, Haake G, Karlsruhe D. Rheological analysis of the stability of pharmaceutical suspensions [Internet]. Vol. V98–154E. 2004. Available from: http://www.rhl.pl/files/17905/Pomiary_i_analiza_stabilnosci_dodatkow_farmaceutycznych.pdf.
Kutschmann E, Gmbh GH, Karlsruhe D. Yield point determination—a critical discussion of different methods [Internet]. 2000. Available from: http://www.rhl.pl/files/17736/Pomiary_punktu_plyniecia.pdf.
Rosin NL, Agabalyan N, Olsen K, Martufi G, Gabriel V, Biernaskie J, et al. Collagen structural alterations contribute to stiffening of tissue after split-thickness skin grafting. Wound Repair Regen [Internet]. 2016;24(2):263–74. Available from: http://doi.wiley.com/10.1111/wrr.12402
Wang XQ, Kravchuk O, Liu PY, Kempf M, Boogaard CVD, Lau P, et al. The evaluation of a clinical scar scale for porcine burn scars. Burns. 2009;35(4):538–46.
Martineau L, Shek PN. Evaluation of a bi-layer wound dressing for burn care: I. Cooling and wound healing properties. Burns. 2006;32(1):70–6.
Caron I, Rossi F, Papa S, Aloe R, Sculco M, Mauri E, et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135–47.
Chen X, Peng LH, Shan YH, Li N, Wei W, Yu L, et al. Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery. Int J Pharm. 2013;447(1–2):171–81.
Guo X, Huang S, Sun J, Wang F. Comparison of the cytotoxicities and wound healing effects of hyaluronan, carbomer, and alginate on skin cells in vitro. Adv Skin Wound Care. 2015;28(9):410–4.
Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen, and wound healing. J Pharmacol Sci. 2009;109(4):525–31.
Huang Y, Meek KM, Ho MW, Paterson CA. Anaylsis of birefringence during wound healing and remodeling following alkali burns in rabbit cornea. Exp Eye Res [Internet]. 2001;73(4):521–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11825023
Le V-H, Lee S, Kim B, Yoon Y, Yoon CJ, Chung WK, et al. Correlation between polarization sensitive optical coherence tomography and second harmonic generation microscopy in skin. Biomed Opt Express [Internet]. 2015;6(7):2542. Available from: https://www.osapublishing.org/boe/abstract.cfm?uri=boe-6-7-2542
Lawrenson JG, Ruskell GL. Investigation of limbal touch sensitivity using a Cochet-Bonnet aesthesiometer. Br J Ophthalmol [Internet]. 1993;77(6):339–43. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=504526&tool=pmcentrez&rendertype=abstract
Boddeda B, Ratna JV, Battu H. A review on mucoadhesive polymers in ophthalmics. Int J Pharm Sci Rev Res. 2014;24(1):237–45.
Yergoz F, Hastar N, Cimenci CE, Ozkan AD, Tekinay T, Guler MO, et al. Heparin mimetic peptide nanofiber gel promotes regeneration of full thickness burn injury. Biomaterials. 2017;134:117–27.
Volkova N, Yukhta M, Pavlovich O, Goltsev A. Application of cryopreserved fibroblast culture with au nanoparticles to treat burns. Nanoscale Res Lett. 2016;11(1):22.
Jin G, Prabhakaran MP, Kai D, Ramakrishna S. Controlled release of multiple epidermal induction factors through core—shell nanofibers for skin regeneration. Eur J Pharm Biopharm. 2013;85(3, part A):689–98.
Acknowledgements
María Florencia Sanchez thanks for the CONICET fellowship. We thank Dr. Paul Hobson, native speaker, for revision of the manuscript.
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, grant number PIP 2013-2015, 11220120100461), the Fondo para la Investigación Científica y Tecnológica (FonCyT, grant number PICT 0173), and the Universidad Nacional de Córdoba (SECYT-UNC, grant number 162/12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sanchez, M.F., Breda, S.A., Soria, E.A. et al. Ciprofloxacin-lidocaine-based hydrogel: development, characterization, and in vivo evaluation in a second-degree burn model. Drug Deliv. and Transl. Res. 8, 1000–1013 (2018). https://doi.org/10.1007/s13346-018-0523-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0523-7