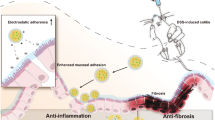
Abstract
Ulcerative colitis (UC) is an inflammatory disease of the colon that severely affects the quality of life of patients and usually responds well to anti-inflammatory agents for symptomatic relief; however, many patients need colectomy, a surgical procedure to remove whole or part of the colon. Though various types of pharmacological agents have been employed for the management of UC, the lack of effectiveness is usually predisposed to various reasons including lack of target-specific delivery of drugs and insufficient drug accumulation at the target site. To overcome these glitches, many researchers have designed and characterized various types of versatile polymeric biomaterials to achieve target-specific delivery of drugs via oral route to optimize their targeting efficiency to the colon, to improve drug accumulation at the target site, as well as to ameliorate off-target effects of chemotherapy. Therefore, the aim of this review was to summarize and critically discuss the pharmaceutical significance and therapeutic feasibility of a wide range of natural and synthetic biomaterials for efficient drug targeting to colon and rationalized treatment of UC. Among various types of biomaterials, natural and synthetic polymer-based hydrogels have shown promising targeting potential due to their innate pH responsiveness, sustained and controlled release characteristics, and microbial degradation in the colon to release the encapsulated drug moieties. These characteristic features make natural and synthetic polymer-based hydrogels superior to conventional pharmacological strategies for the management of UC.








Similar content being viewed by others
References
Danese S, Fiocchi C. Ulcerative colitis. N. Engl. J. Med. 2011;365:1713–25. https://doi.org/10.1055/s-0030-1268248.
Cosnes J, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. YGAST. 2011;140:1785–1794.e4. https://doi.org/10.1053/j.gastro.2011.01.055.
B. Moum, A. Ekbom, M.H. Vatn, K. Elgjo, Change in the extent of colonoscopic and histological involvement in ulcerative colitis over time, 94 (1999) 0–5.
E. Langholz, Current trends in inflammatory bowel disease: the natural history, (2010) 77–86. doi:https://doi.org/10.1177/1756283X10361304, 3
M. Van Der Have, H.H. Fidder, M. Leenders, A.A. Kaptein, M.E. Van Der Valk, A.A. Van Bodegraven, G. Dijkstra, D.J. De Jong, M. Pierik, C.Y. Ponsioen, A.E.V.D.M. Jong, C.J. Van Der Woude, P.C. Van De Meeberg, M.J.L. Romberg-camps, J.R. Vermeijden, P.D. Siersema, B. Oldenburg, Self-reported disability in patients with inflammatory bowel disease largely determined by disease activity and illness perceptions, 21 (2015) 369–377. doi:https://doi.org/10.1097/MIB.0000000000000278.
L. Zhao, J. Li, T. Yu, G. Chen, Y. Yuan, Q. Chen, 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis, 9 (2014). doi:https://doi.org/10.1371/journal.pone.0094208.
Xu J, Tam M, Samadei S, Lerouge S, Barralet J, Stevenson M, et al. Department of Mining and Materials Engineering, Faculty of Engineering, McGill Marta Cerruti, PhDMucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2016; https://doi.org/10.1016/j.actbio.2016.10.026.
Duan H, Lü S, Gao C, Bai X, Qin H, Wei Y, et al. Mucoadhesive microparticulates based on polysaccharide for target dual drug delivery of 5-aminosalicylic acid and curcumin to inflamed colon. Colloids Surfaces B Biointerfaces. 2016;145:510–9. https://doi.org/10.1016/j.colsurfb.2016.05.038.
Sales-Campos H, Basso PJ, Alves VBF, Fonseca MTC, Bonfá G, Nardini V, et al. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96–107. https://doi.org/10.1590/1414-431X20143774.
Xu J, Tam M, Samaei S, Lerouge S, Barralet J, Stevenson MM, et al. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017;48:247–57. https://doi.org/10.1016/j.actbio.2016.10.026.
Panccione R, Ghosh S, Middleton S, Marquez JR, Khalif I, Flint L, et al. Infliximab, azathioprine, or infliximab+ azathioprine for treatment of moderate to severe ulcerative colitis: the UC SUCCESS trial. Gastroenterology. 2011;140:S-134.
Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D’Inca R, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30–5. https://doi.org/10.1016/j.cgh.2010.09.026.
Smits P, Thien T, Erkelens DW, Romijn JA. The Netherlands Journal of Medicine. 2004;
ASHP. No title. In: AHFS; 1994.
Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–77. https://doi.org/10.1111/apt.12616.
Ahmed O, Nguyen GC. Therapeutic challenges of managing inflammatory bowel disease in the elderly patient. Expert Rev. Gastroenterol. Hepatol. 2016;10:1005–10. https://doi.org/10.1080/17474124.2016.1179579.
Shilpa, Srinivasan BP, Chauhan M. Niosomes as vesicular carriers for delivery of proteins and biologicals. Int. J. Drug Deliv. 2011;3:14–24. https://doi.org/10.5138/ijdd.2010.0975.0215.03050.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. https://doi.org/10.1016/j.addr.2012.09.037.
Çağdaş M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery. Nanotechnol. Nanomater. “Application Nanotechnol. Drug Deliv.”. 2014:1–50. https://doi.org/10.5772/57028.
Aleksovski A, Dreu R, Gašperlin M, Planinšek O. Mini-tablets: a contemporary system for oral drug delivery in targeted patient groups. Expert Opin Drug Deliv. 2015;12:65–84. https://doi.org/10.1517/17425247.2014.951633.
Dash TR, Verma P. Matrix tablets: an approach towards oral extended release drug delivery. Int J Pharma Res Rev. 2013;2:12–24.
Fox CB, Kim J, Le LV, Nemeth CL, Chirra HD, Desai TA. Micro/nanofabricated platforms for oral drug delivery. J Control Release. 2015;219:431–44. https://doi.org/10.1016/j.jconrel.2015.07.033.
Luo Y, Wang Q. Zein-based micro- and nano-particles for drug and nutrient delivery: a review. J Appl Polym Sci. 2014;131 https://doi.org/10.1002/app.40696.
S. Zhang, J. Ermann, M.D. Succi, A. Zhou, M.J. Hamilton, B. Cao, J.R. Korzenik, J.N. Glickman, P.K. Vemula, L.H. Glimcher, G. Traverso, R. Langer, J.M. Karp, An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease, Sci Transl Med 7 (2015) 300ra128-300ra128. doi:https://doi.org/10.1126/scitranslmed.aaa5657.
Toma H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012:2193–221. https://doi.org/10.1039/c1cs15203c.
Shi J, Xing MMQ, Zhong W. Development of hydrogels and biomimetic regulators as tissue engineering scaffolds. Membranes (Basel). 2012;2:70–90. https://doi.org/10.3390/membranes2010070.
Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer (Guildf). 2008;49:1993–2007. https://doi.org/10.1016/j.polymer.2008.01.027.
Sohail M, Ahmad M, Usman M, Ali L. Controlled delivery of valsartan by cross-linked polymeric matrices: synthesis, in vitro and in vivo evaluation. Int J Pharm. 2015;487:110–9. https://doi.org/10.1016/j.ijpharm.2015.04.013.
Jiang J, Parker CE, Fuller JR, Kawula TH, Borchers CH. NIH public access. Anal Chim Acta. 2008;605:70–9. https://doi.org/10.1016/j.immuni.2010.12.017.Two-stage.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Publ Gr. 2016;1:1–18. https://doi.org/10.1038/natrevmats.2016.71.
Long Zhao HM, Yosh F. Synthesis of pH-sensitive and biodegradable CM-cellulose/chitosan polyampholytic hydrogels with electron beam irradiation. J Bioact Compat Polym. 2008;23:319–33. https://doi.org/10.1177/0883911508092302.
Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J. 2015;65:252–67. https://doi.org/10.1016/j.eurpolymj.2014.11.024.
Neufeld L, Bianco-Peled H. Accepted crtInt J Biol Macromol. 2017;101:852–61. https://doi.org/10.1016/j.ijbiomac.2017.03.167.
Rehmani S, Ahmad M, Usman M, Hina M. Development of natural and synthetic polymer-based semi-interpenetrating polymer network for controlled drug delivery: optimization and in vitro evaluation studies. Polym Bull. 2016;74:737–61. https://doi.org/10.1007/s00289-016-1743-y.
Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. https://doi.org/10.1016/j.addr.2009.07.019.
Langer R, Vacanti J. Tissue engineering. Am. Assoc. Adv Sci. 1993;260:920–6. https://doi.org/10.1016/j.biomaterials.2009.08.058.Thermosensitive.
Gumera C, Rauck B, Wang Y. Materials for central nervous system regeneration: bioactive cues. J Mater Chem. 2011;21:7033. https://doi.org/10.1039/c0jm04335d.
Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30:3371–7. https://doi.org/10.1016/j.biomaterials.2009.03.030.
Tae G, Kim YJ, Il Choi W, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979–86. https://doi.org/10.1021/bm0701189.
Lü S, Li B, Ni B, Sun Z, Liu M, Wang Q. Thermoresponsive injectable hydrogel for three-dimensional cell culture: chondroitin sulfate bioconjugated with poly(N-isopropylacrylamide) synthesized by RAFT polymerization. Soft Matter. 2011;7:10763. https://doi.org/10.1039/c1sm06053h.
Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–56. https://doi.org/10.1016/j.biomaterials.2010.02.044.Bioactive.
Buwalda SJ, Boere KWM, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release. 2014;190:254–73. https://doi.org/10.1016/j.jconrel.2014.03.052.
Lohani A, Singh G, Bhattacharya SS, Verma A. Interpenetrating polymer networks as innovative drug delivery systems. J Drug Deliv. 2014;2014:583612–1. https://doi.org/10.1155/2014/583612.
Chatterjee B, Amalina N, Sengupta P, Mandal UK. Mucoadhesive polymers and their mode of action: a recent update, vol. 7; 2017. p. 195–203. https://doi.org/10.7324/JAPS.2017.70533.
Dheer D, Arora D, Jaglan S, Rawal RK, Shankar R, Dheer D, et al. Polysaccharides based nanomaterials for targeted anti-cancer drug delivery, vol. 2330; 2017. https://doi.org/10.3109/1061186X.2016.1172589.
An B, Lin YS, Brodsky B. Collagen interactions: drug design and delivery. Adv Drug Deliv Rev. 2016;97:69–84. https://doi.org/10.1016/j.addr.2015.11.013.
Ahmad E, Fatima MT, Hoque M, Owais M, Saleemuddin M. Fibrin matrices: the versatile therapeutic delivery systems. Int J Biol Macromol. 2015;81:121–36. https://doi.org/10.1016/j.ijbiomac.2015.07.054.
Van Tomme SR, Hennink WE. Biodegradable dextran hydrogels for protein delivery applications. Expert Rev Med Devices. 2007;4:147–64. https://doi.org/10.1586/17434440.4.2.147.
Kolanthai E, Ganesan K, Epple M, Kalkura SN. Synthesis of nanosized hydroxyapatite/agarose powders for bone filler and drug delivery application. Mater Today Commun. 2016;8:31–40. https://doi.org/10.1016/j.mtcomm.2016.03.008.
Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Peptide-based stimuli-responsive biomaterials. Soft Matter. 2006;2:822. https://doi.org/10.1039/b607706d.
MacLeod GS, Fell JT, Collett JH. Studies on the physical properties of mixed pectin/ethylcellulose films intended for colonic drug delivery. Int J Pharm. 1997;157:53–60. https://doi.org/10.1016/S0378-5173(97)00216-0.
Semdé R, Amighi K, Pierre D, Devleeschouwer MJ, Moës AJ. Leaching of pectin from mixed pectin/insoluble polymer films intended for colonic drug delivery. Int J Pharm. 1998;174:233–41. https://doi.org/10.1016/S0378-5173(98)00269-5.
Wong TW, Nurjaya S. Drug release property of chitosan-pectinate beads and its changes under the influence of microwave. Eur J Pharm Biopharm. 2008;69:176–88. https://doi.org/10.1016/j.ejpb.2007.09.015.
Thakur BR, Singh RK, Handa AK, Rao MA. Critical reviews in food science and nutrition chemistry and uses of pectin—a review chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr. 2009;37(1):47–73. https://doi.org/10.1080/10408399709527767.
Bawa P, Choonara YE, Du Toit LC, Kumar P, Ndesendo VMK, Meyer LCR, et al. A novel stimuli-synchronized alloy-treated matrix for space-defined gastrointestinal delivery of mesalamine in the large white pig model. J Control Release. 2013;166:234–45. https://doi.org/10.1016/j.jconrel.2012.12.011.
Bigucci F, Luppi B, Monaco L, Cerchiara T, Zecchi V. Pectin-based microspheres for colon-specific delivery of vancomycin. J Pharm Pharmacol. 2009;61:41–6. https://doi.org/10.1211/jpp/61.01.0006.
Eswaramma S, Reddy NS, Rao KSVK. Phosphate crosslinked pectin based dual responsive hydrogel networks and nanocomposites: development, swelling dynamics and drug release characteristics. Int J Biol Macromol. 2017;103:1162–72. https://doi.org/10.1016/j.ijbiomac.2017.05.160.
Almeida EAMS, Bellettini IC, Garcia FP, Farinácio MT, Nakamura CV, Rubira AF, et al. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr Polym. 2017;171:259–66. https://doi.org/10.1016/j.carbpol.2017.05.034.
R.K. Mishra, M. Datt, A.K. Banthia, Synthesis and characterization of pectin/PVP hydrogel membranes for drug delivery system, 9 (2008). doi:https://doi.org/10.1208/s12249-008-9048-6.
L. Costas, L.M. Pera, A.G. López, M. Mechetti, G.R. Castro, Controlled release of sulfasalazine release from “smart” pectin gel microspheres under physiological simulated fluids, (2012) 1396–1407. doi:https://doi.org/10.1007/s12010-012-9615-x.
Newton AMJ, Lakshmanan P. Effect of HPMC-E15 LV premium polymer on release profile and compression characteristics of chitosan/ pectin colon targeted mesalamine matrix tablets and in vitro study on effect of pH impact on the drug release profile. Recent Pat Drug Deliv Formul. 2014;8:46–62. https://doi.org/10.2174/1872211308666140225143926.
Birch NP, Barney LE, Pandres E, Peyton SR, JD. Thermal-responsive behavior of a cell compatible chitosan/pectin hydrogel Schiffman. Biomacromolecules. 2015;16(6);1837–1843
Ekaterina D, Sergey AM. Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr Polym. 2016;157:9–20. https://doi.org/10.1016/j.carbpol.2016.09.048.
Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–84. https://doi.org/10.1021/cr030441b.
J.J. Thevarajah, Characterisation of chitosan and its films, (2016).
Aider M. Chitosan application for active bio-based films production and potential in the food industry: review. LWT - Food Sci Technol. 2010;43:837–42. https://doi.org/10.1016/j.lwt.2010.01.021.
Muzzarelli RAA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci. 1997;53:131–40. https://doi.org/10.1007/PL00000584.
No HK, Young Park N, Ho Lee S, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74:65–72. https://doi.org/10.1016/S0168-1605(01)00717-6.
Ravi Kumar MN. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27. https://doi.org/10.1016/S1381-5148(00)00038-9.
Borchard G, Lueßen HL, De Boer AG, Verhoef JC, Lehr CM, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Release. 1996;39:131–8. https://doi.org/10.1016/0168-3659(95)00146-8.
S.T. Lim, B. Forbes, G.P. Martin, M.B. Brown, In vivo and in vitro characterization of novel microparticulates based on hyaluronan and chitosan hydroglutamate, 2 (2001).
Aguzzi C, Ortega A, Bonferoni MC, Sandri G, Cerezo P, Salcedo I, et al. Assessement of anti-inflammatory properties of microspheres prepared with chitosan and 5-amino salicylic acid over inflamed Caco-2 cells. Carbohydr Polym. 2011;85:638–44. https://doi.org/10.1016/j.carbpol.2011.03.027.
Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2012;64:49–60. https://doi.org/10.1016/j.addr.2012.09.024.
Bukhari SMH, Khan S, Rehanullah M, Ranjha NM. Synthesis and characterization of chemically cross-linked acrylic acid/gelatin hydrogels: effect of pH and composition on swelling and drug release. Int J Polym Sci. 2015;2015:1–15. https://doi.org/10.1155/2015/187961.
Duan H, Lu S, Qin H, Gao C, Bai X, Wei Y, et al. Co-delivery of zinc and 5-aminosalicylic acid from alginate/N-succinyl-chitosan blend microspheres for synergistic therapy of colitis. Int J Pharm. 2017;516:214–24. https://doi.org/10.1016/j.ijpharm.2016.11.036.
Wang QS, Wang GF, Zhou J, Gao LN, Cui YL. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharm. 2016;515:176–85. https://doi.org/10.1016/j.ijpharm.2016.10.002.
Ramadass SK, Perumal S, Jabaris SL, Madhan B. Preparation and evaluation of mesalamine collagen in situ rectal gel: a novel therapeutic approach for treating ulcerative colitis. Eur J Pharm Sci. 2013;48:104–10. https://doi.org/10.1016/j.ejps.2012.10.015.
Jr SBA, Nettles DL, Ph D, Setton LA. Author manuscript. J Biomed Mater Res Part B Appl Biomater. 2010:1–14. https://doi.org/10.1002/jbm.b.31289.Release.
Mura C, Nácher A, Merino V, Merino-Sanjuan M, Carda C, Ruiz A, et al. N-Succinyl-chitosan systems for 5-aminosalicylic acid colon delivery: in vivo study with TNBS-induced colitis model in rats. Int J Pharm. 2011;416:145–54. https://doi.org/10.1016/j.ijpharm.2011.06.025.
Bai XY, Yan Y, Wang L, Zhao LG, Wang K. Novel pH-sensitive hydrogels for 5-aminosalicylic acid colon targeting delivery: in vivo study with ulcerative colitis targeting therapy in mice. Drug Deliv. 2015;0:1–7. https://doi.org/10.3109/10717544.2014.996924.
Ju E, Park K, Su K, Kim J, Yang J, Kong J, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release. 2010;141:2–12. https://doi.org/10.1016/j.jconrel.2009.09.010.
Kim KS, Park SJ, Yang J, Jeon J, Bhang SH, Kim B, et al. Acta Biomaterialia injectable hyaluronic acid–tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011;7:666–74. https://doi.org/10.1016/j.actbio.2010.09.030.
J. Kim, I. Sook, T. Hyung, K. Back, S. Jung, G. Tae, I. Noh, S. Hoon, Y. Park, K. Sun, Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells, 28 (2007) 1830–1837. doi:https://doi.org/10.1016/j.biomaterials.2006.11.050.
Jung HH, Park K, Han DK. Preparation of TGF-β 1-conjugated biodegradable pluronic F127 hydrogel and its application with adipose-derived stem cells. J Control Release. 2010;147:84–91. https://doi.org/10.1016/j.jconrel.2010.06.020.
Manuscript A, Cells S, Hyaluronic U, Crosslinked D. NIH public access, vol. 32; 2012. p. 2466–78. https://doi.org/10.1016/j.biomaterials.2010.12.024.Controlling.
Xiao B, Zhang Z, Viennois E, Kang Y, Zhang M, Han MK. Combination therapy for ulcerative colitis: orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics. 2016;6 https://doi.org/10.7150/thno.15710.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114:1–14. https://doi.org/10.1016/j.jconrel.2006.04.017.
Grellier M, Granja PL, Fricain JC, Bidarra SJ, Renard M, Bareille R, et al. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials. 2009;30:3271–8. https://doi.org/10.1016/j.biomaterials.2009.02.033.
K.M. Dupont, J.D. Boerckel, N. Huebsch, NIH public access, east 32 (2012) 65–74. doi:https://doi.org/10.1016/j.biomaterials.2010.08.074.An.
Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, et al. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials. 2010;31:7012–20. https://doi.org/10.1016/j.biomaterials.2010.05.078.
Dawlee S, Sugandhi A, Balakrishnan B, Labarre D, Jayakrishnan A. Oxidized chondroitin sulfate-cross-linked gelatin matrixes: a new class of hydrogels. Biomacromolecules. 2005;6:2040–8. https://doi.org/10.1021/bm050013a.
Barkat K, Ahmad M, Minhas MU, Khalid I. Oxaliplatin-loaded crosslinked polymeric network of chondroitin sulfate-co-poly(methacrylic acid) for colorectal cancer: its toxicological evaluation. J Appl Polym Sci. 2017;45312:45312. https://doi.org/10.1002/app.45312.
You YC, Dong LY, Dong K, Xu W, Yan Y, Zhang L, et al. In vitro and in vivo application of pH-sensitive colon-targeting polysaccharide hydrogel used for ulcerative colitis therapy. Carbohydr Polym. 2015;130:243–53. https://doi.org/10.1016/j.carbpol.2015.03.075.
Birch NP, Birch NP, Barney LE, Pandres E, Peyton SR, Schi JD. Thermal-responsive behavior of a cell compatible chitosan/pectin hydrogel; 2015. https://doi.org/10.1021/acs.biomac.5b00425.
Ondeck MG, Engler AJ. Mechanical characterization of a dynamic and tunable methacrylated hyaluronic acid hydrogel. J Biomech Eng. 2016;138:21003. https://doi.org/10.1115/1.4032429.
Hu J, Li HY, Williams GR, Yang HH, Tao L, Zhu LM. Electrospun poly(N-isopropylacrylamide)/ethyl cellulose nanofibers as thermoresponsive drug delivery systems. J Pharm Sci. 2016;105:1104–12. https://doi.org/10.1016/S0022-3549(15)00191-4.
Feng H, Zhang L, Zhu C. Genipin crosslinked ethyl cellulose-chitosan complex microspheres for anti-tuberculosis delivery. Colloids Surf B Biointerfaces. 2013;103:530–7. https://doi.org/10.1016/j.colsurfb.2012.11.007.
Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Ther Deliv. 2015;6:41–58. https://doi.org/10.4155/tde.14.91.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3:1377–97. https://doi.org/10.3390/polym3031377.
Ostroha JL, Lowman A, Dan N. PEG-based degradable networks for drug delivery applications. Chem Biol Eng Doctor of. 2006;165 http://hdl.handle.net/1860/842
Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG—a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81. https://doi.org/10.1016/j.jconrel.2014.06.046.
Zhao L, Liu M, Wang J, Zhai G. Chondroitin sulfate-based nanocarriers for drug/gene delivery. Carbohydr Polym. 2015;133:391–9. https://doi.org/10.1016/j.carbpol.2015.07.063.
Ramasamy T, Khandasamy US, Shanmugam S, Ruttala H. Formulation and evaluation of chondroitin sulphate tablets of aceclofenac for colon targeted drug delivery, Iran. J Pharm Res. 2012;11:465–79. https://doi.org/10.1590/S1984-82502011000200011.
Davaran S, Rashidi MR, Khani A. Synthesis of chemically cross-linked hydroxypropyl methyl cellulose hydrogels and their application in controlled release of 5-amino salicylic acid. Drug Dev Ind Pharm. 2007;33:881–7. https://doi.org/10.1080/03639040601150278.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48:139–57. https://doi.org/10.1016/S0169-409X(01)00112-0.
Dasgupta A, Mondal JH, Das D. Peptide hydrogels. RSC Adv. 2013;3:9117–49. https://doi.org/10.1039/c3ra40234g.
Aytac Z, Sen HS, Durgun E, Uyar T. Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf B Biointerfaces. 2015;128:331–8. https://doi.org/10.1016/j.colsurfb.2015.02.019.
S. Poh, J.B. Lin, A. Panitch, Release of anti-inflammatory peptides from thermosensitive nanoparticles with degradable cross-links suppresses pro-inflammatory cytokine production, (2015). doi:https://doi.org/10.1021/bm501849p.
Singh B, Sharma N, Chauhan N. Synthesis, characterization and swelling studies of pH responsive psyllium and methacrylamide based hydrogels for the use in colon specific drug delivery. Carbohydr Polym. 2007;69:631–43. https://doi.org/10.1016/j.carbpol.2007.01.020.
Bezzio C, Fascì-Spurio F, Viganò C, Meucci G, Papi C, Saibeni S. The problem of adherence to therapy in ulcerative colitis and the potential utility of multi-matrix system (MMX) technology. Expert Rev Gastroenterol Hepatol. 2017;11:33–41. https://doi.org/10.1080/17474124.2017.1256200.
Acknowledgements
The authors would like to acknowledge the Higher Education Commission of Pakistan for the financial support through Project No. 21-487/SRGP/R&D/HEC/2015. This study became possible due to the support of the Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors reported that they have no conflict of interest in the present research work.
Rights and permissions
About this article
Cite this article
Sohail, M., Mudassir, Minhas, M.U. et al. Natural and synthetic polymer-based smart biomaterials for management of ulcerative colitis: a review of recent developments and future prospects. Drug Deliv. and Transl. Res. 9, 595–614 (2019). https://doi.org/10.1007/s13346-018-0512-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0512-x