Abstract
Background and Objective
p75ECD-Fc is a novel antagonist of toxic amyloid beta protein and other neurodegenerative factors with potential for the treatment of Alzheimer’s disease (AD). Preclinical studies showed that it can alleviate the AD pathologies in animal models of dementia. In a previous paper, we used non-compartmental pharmacokinetic analysis to obtain preliminary pharmacokinetic data for p75ECD-Fc in Sprague Dawley (SD) rats. We also studied the tissue distribution in terms of drug metabolism that helped us to understand possible mechanisms of action. Here, we aim to develop population pharmacokinetic models that can describe the pharmacokinetics of p75ECD-Fc in serum and tissues.
Methods
p75ECD-Fc was delivered to SD rats via two routes (intravenous and subcutaneous) at a single dose of 3 mg/kg (n = 15). Blood (n = 12) and tissue samples (n = 10–15) were then separated at different time points for a total duration of 42 days post dosage. The concentration of p75ECD-Fc in serum and tissues was measured using an enzyme-linked immunosorbent assay.
Results
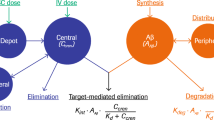
Data were best fitted to a 2-compartment model with linear elimination kinetics. The population parameter estimates for clearance, and volume of central and peripheral compartments were 0.000176 L/h, 0.0145 L and 0.0263 L, respectively. The presence of anti-drug antibodies was added to the final model as a covariate on clearance. The subcutaneous bioavailability was estimated to be 53.5% with a first-order absorption rate constant of 0.00745 1/h. By modeling of individual tissue concentrations, p75ECD-Fc was found to exhibit modest tissue distribution with estimated tissue/plasma partition coefficients (R) ranging from 0.004 to 0.2.
Conclusion
This is the first report of a pharmacokinetic model for p75ECD-Fc and these results may facilitate the ongoing development of p75ECD-Fc and translation to clinical studies.









Similar content being viewed by others
References
Alzheimer's Disease International: World Alzheimer Report 2018; The state of the art of dementia research: new frontiers. 2018.
Alzheimer's association: FDA-approved treatments for Alzheimer’s. 2019.
Khoury R, Rajamanickam J, Grossberg GT. An update on the safety of current therapies for Alzheimer’s disease: focus on rivastigmine. Ther Adv Drug Saf. 2018;9(3):171–8. https://doi.org/10.1177/2042098617750555.
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. https://doi.org/10.1126/science.1072994.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59.
Zhou X-F. The imbalance of neurotrophic signalling: an alternate hypothesis for the pathogenesis and drug development of Alzheimer’s disease. Proc Neurosci. 2016;1(1):13–8.
Cattaneo A, Calissano P. Nerve growth factor and Alzheimer’s disease: new facts for an old hypothesis. Mol Neurobiol. 2012;46(3):588–604. https://doi.org/10.1007/s12035-012-8310-9.
Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J Neurochem. 2006;97(2):475–87. https://doi.org/10.1111/j.1471-4159.2006.03764.x.
Wang YJ, Valadares D, Sun Y, Wang X, Zhong JH, Liu XH, et al. Effects of proNGF on neuronal viability, neurite growth and amyloid-beta metabolism. Neurotox Res. 2010;17(3):257–67. https://doi.org/10.1007/s12640-009-9098-x.
Ibáñez CF, Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35(7):431–40. https://doi.org/10.1016/j.tins.2012.03.007.
Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, et al. Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. J Clin Invest. 1997;100(9):2333–40. https://doi.org/10.1172/JCI119772.
Mamada N, Tanokashira D, Hosaka A, Kametani F, Tamaoka A, Araki W. Amyloid β-protein oligomers upregulate the β-secretase, BACE1, through a post-translational mechanism involving its altered subcellular distribution in neurons. Mol Brain. 2015;8(1):73. https://doi.org/10.1186/s13041-015-0163-5.
Zhou XF, Wang YJ. The p75NTR extracellular domain. Prion. 2011;5:161–3. https://doi.org/10.4161/pri.5.3.16896.
Yao XQ, Jiao SS, Saadipour K, Zeng F, Wang QH, Zhu C, et al. p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-beta toxicity in the brain of Alzheimer’s disease. Mol Psychiatry. 2015;20(11):1301–10. https://doi.org/10.1038/mp.2015.49.
Wang YJ, Wang X, Lu JJ, Li QX, Gao CY, Liu XH, et al. p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain. J Neurosci. 2011;31(6):2292–304. https://doi.org/10.1523/JNEUROSCI.2733-10.2011.
Wang Q-H, Wang Y-R, Zhang T, Jiao S-S, Liu Y-H, Zeng F, et al. Intramuscular delivery of p75NTR ectodomain by an AAV vector attenuates cognitive deficits and Alzheimer’s disease-like pathologies in APP/PS1 transgenic mice. J Neurochem. 2016;138(1):163–73. https://doi.org/10.1111/jnc.13616.
Enbrel. Pharmaceuticals. Amgen Inc. and Wyeth-Ayerst, Thousand Oaks. 2004.
Kelliny S, Lam HY, Parikh A, Wang Y, Bobrovskaya L, Upton R, et al. Preclinical study of the pharmacokinetics of p75ECD-Fc, a novel human recombinant protein for treatment of Alzheimer’s disease, in Sprague Dawley rats. Curr Drug Metab. 2020;21:1–10. https://doi.org/10.2174/1389200221666200502015203.
Lin JH. Pharmacokinetics of biotech drugs: peptides, proteins and monoclonal antibodies. Curr Drug Metab. 2009;10(7):661–91. https://doi.org/10.2174/138920009789895499.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. 2017.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–23. https://doi.org/10.1109/TAC.1974.1100705.
Probst RJ, Lim JM, Bird DN, Pole GL, Sato AK, Claybaugh JR. Gender differences in the blood volume of conscious Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2006;45(2):49–52.
Factors affecting the lymphatic absorption of macromolecules following extravascular administration. Pharmaceutical Research (New York). 1996;13((New York):S396-S.
McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2(1):89–96. https://doi.org/10.1016/j.ddtec.2005.05.006.
Kagan L, Gershkovich P, Mendelman A, Amsili S, Ezov N, Hoffman A. The role of the lymphatic system in subcutaneous absorption of macromolecules in the rat model. Eur J Pharm Biopharm. 2007;67(3):759–65. https://doi.org/10.1016/j.ejpb.2007.04.002.
Wang W, Chen N, Shen X, Cunningham P, Fauty S, Michel K, et al. Lymphatic transport and catabolism of therapeutic proteins after subcutaneous administration to rats and dogs. Drug Metab Dispos. 2012;40(5):952–62. https://doi.org/10.1124/dmd.111.043604.
Chen X, DuBois DC, Almon RR, Jusko WJ. Biodistribution of etanercept to tissues and sites of inflammation in arthritic rats. Drug Metab Dispos. 2015a;43(6):898–907. https://doi.org/10.1124/dmd.114.062901.
Lee B-y, Kwon K-i, Kim M-S, Baek I-h. Michaelis–Menten elimination kinetics of etanercept, rheumatoid arthritis biologics, after intravenous and subcutaneous administration in rats. Eur J Drug Metab Pharmacokinet. 2016;41(4):433-9. https://doi.org/10.1007/s13318-015-0270-9.
Hall MP. Biotransformation and in vivo stability of protein biotherapeutics: impact on candidate selection and pharmacokinetic profiling. Drug Metab Dispos. 2014;42(11):1873–80. https://doi.org/10.1124/dmd.114.058347.
Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 2010;184(4):1968–76. https://doi.org/10.4049/jimmunol.0903296.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. https://doi.org/10.1038/nri2155.
Gómez-Mantilla JD, Trocóniz IF, Parra-Guillén Z, Garrido MJ. Review on modeling anti-antibody responses to monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2014;41(5):523–36. https://doi.org/10.1007/s10928-014-9367-z.
Kamath AV. Translational pharmacokinetics and pharmacodynamics of monoclonal antibodies. Drug Discov Today Technol. 2016;21–22:75–83. https://doi.org/10.1016/j.ddtec.2016.09.004.
Chen X, DuBois DC, Almon RR, Jusko WJ. Biodistribution of etanercept to tissues and sites of inflammation in arthritic rats. Drug Metab Dispos. 2015b;43(6):898–907. https://doi.org/10.1124/dmd.114.062901.
Conner KP, Rock BM, Kwon GK, Balthasar JP, Abuqayyas L, Wienkers LC, et al. Evaluation of near infrared fluorescent labeling of monoclonal antibodies as a tool for tissue distribution. Drug Metab Dispos. 2014;42(11):1906–13. https://doi.org/10.1124/dmd.114.060319.
Cohen R, Stammes MA, de Roos IH, Stigter-van Walsum M, Visser GW, van Dongen GA. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011;1(1):31. https://doi.org/10.1186/2191-219X-1-31.
Liu Y-H, Wang Y-R, Xiang Y, Zhou H-D, Giunta B, Manucat N, et al. Clearance of amyloid-beta in Alzheimer’s disease: shifting the action site from center to periphery. Mol Neurobiol. 2014. https://doi.org/10.1007/s12035-014-8694-9.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by NHMRC grants (APP1020567, APP1021409) and a supporting grant from the University of South Australia. The authors also acknowledge the support provided to S. Kelliny by a Commonwealth Research and Training scholarship and the Egyptian Ministry of Higher Education. p75ECD-Fc was provided by Fujian Tiantai Medical Technology Ltd.
Conflict of interest
Xin-Fu Zhou is one of the inventors of p75ECD-Fc for the treatment of AD. The other authors declare no conflict of interest.
Ethical approval
All animal procedures performed in this study were approved by the Animal Ethics Committee (AEC) of the University of South Australia (approval number U14/16) and in compliance with the South Australian Animal Welfare Act 1985 and Regulations 2012.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data are available on negotiation with the authors.
Code availability
Code are available on negotiation with the authors.
Author Contributions
All authors participated in the study design and/or data interpretation. S.K. conducted the animal study and samples analysis. Statistical analysis, model development and evaluation were performed by R.U. and S.K. The first draft of the manuscript was written by S.K. and R.U. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kelliny, S., Bobrovskaya, L., Zhou, XF. et al. Pharmacokinetic Modelling of Human Recombinant Protein, p75ECD-Fc: A Novel Therapeutic Approach for Treatment of Alzheimer’s Disease, in Serum and Tissue of Sprague Dawley Rats. Eur J Drug Metab Pharmacokinet 46, 235–248 (2021). https://doi.org/10.1007/s13318-020-00662-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00662-0