Abstract
Background and Objective
Tacrolimus, a major immunosuppressant used after transplantation, is associated with large interindividual variation involving genetic polymorphisms in metabolic processes. A common variant of the cytochrome P450 (CYP) 3A5 gene, CYP3A5*3, affects blood concentrations of tacrolimus. However, tacrolimus pharmacokinetics at the early stage of transplantation have not been adequately studied in heart transplantation. We retrospectively examined the impact of the CYP3A5 genotype on tacrolimus pharmacokinetics at the early stage of heart transplantation.
Methods
The tacrolimus pharmacokinetic profile was obtained from 65 patients during the first 5 weeks after heart transplantation. Differences in the patients’ characteristics and tacrolimus pharmacokinetic parameters between the CYP3A5 expresser (*1/*1 or *1/*3 genotypes) and non-expresser (*3/*3 genotype) groups were assessed by the Chi-square test, Student’s t test, or Mann–Whitney U test.
Results
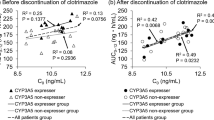
The CYP3A5 *1/*1, *1/*3, and *3/*3 genotypes were detected in 5, 22, and 38 patients, respectively. All patients started clotrimazole therapy approximately 1 week after starting tacrolimus. Apparent clearance and dose/weight to reach the target trough concentration (C0) were significantly higher in the expresser group than in the non-expresser group (0.32 vs. 0.19 L/h/kg, p = 0.0003; 0.052 vs. 0.034 mg/kg/day, p = 0.0002); there were no significant differences in the area under the concentration-time curve from 0 to 12 h (AUC0–12) and concentrations at any sampling time point between the two groups.
Conclusion
Similar concentration–time curves for tacrolimus were obtained in the expresser and non-expresser groups by dose adjustment based on therapeutic drug monitoring. These results demonstrate the importance of the CYP3A5 genotype in tacrolimus dose optimization based on therapeutic drug monitoring after heart transplantation.

Similar content being viewed by others
References
Margreiter R. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation a randomised multicentre study. Lancet. 2002;359:741–6.
Böttiger Y, Brattström C, Tydén G, Säwe J, Groth C. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol. 1999;48(3):445–8.
Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16(9):1905–9.
Wong S. Therapeutic drug monitoring for immunosuppressants. Clin Chim Acta. 2001;313(1–2):241–53.
Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78(8):1182–7.
Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos Biol Fate Chem. 2006;34(5):836–47.
Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos Biol Fate Chem. 1992;20(5):753–61.
Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007;22(5):328–35.
Dai Y, Hebert M, Isoherranen N, Davis C, Marsh C, Shen D, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34(5):836–47.
Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet Genom. 2006;16(2):119–27.
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–91.
Staatz C, Goodman L, Tett S. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141–75.
Diaz-Molina B, Tavira B, Lambert JL, Bernardo MJ, Alvarez V, Coto E. Effect of CYP3A5, CYP3A4, and ABCB1 genotypes as determinants of tacrolimus dose and clinical outcomes after heart transplantation. Transplant Proc. 2012;44(9):2635–8.
Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, et al. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30(12):1352–9.
Kniepeiss D, Renner W, Trummer O, Wagner D, Wasler A, Khoschsorur GA, et al. The role of CYP3A5 genotypes in dose requirements of tacrolimus and everolimus after heart transplantation. Clin Transplant. 2011;25(1):146–50.
Lesche D, Sigurdardottir V, Setoud R, Oberhänsli M, Carrel T, Fiedler G, et al. CYP3A5*3 and POR*28 genetic variants influence the required dose of tacrolimus in heart transplant recipients. Ther Drug Monit. 2014;36(6):710–5.
Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P, et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3(4):477–83.
Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2001;16(9):1905–9.
Cangemi G, Barco S, Bonifazio P, Maffia A, Agazzi A, Melioli G. Comparison of antibody-conjugated magnetic immunoassay and liquid chromatography-tandem mass spectrometry for the measurement of cyclosporine and tacrolimus in whole blood. Int J Immunopathol Pharmacol. 2013;26(2):419–26.
Pfeffer M. Estimation of mean residence time from data obtained when multiple-dosing steady state has been reached. J Pharm Sci. 1984;73(6):854–6.
Choy M. Tacrolimus interaction with clotrimazole: a concise case report and literature review. P T Peer Rev J Formul Manag. 2010;35(10):568–9.
El-Asmar J, Gonzalez R, Bookout R, Mishra A, Kharfan-Dabaja MA. Clotrimazole troches induce supratherapeutic blood levels of sirolimus and tacrolimus in an allogeneic hematopoietic cell-transplant recipient resulting in acute kidney injury. Hematol Oncol Stem Cell Ther. 2016;9(4):157–61.
Gibbs M, Kunze K, Howald W, Thummel K. Effect of inhibitor depletion on inhibitory potency: tight binding inhibition of CYP3A by clotrimazole. Drug Metab Dispos. 1999;27(5):596–9.
Gibbs M, Thummel K, Shen D, Kunze K. Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos. 1999;27(2):180–7.
Laftavi MR, Pankewycz O, Patel S, Nader N, Kohli R, Feng L, et al. African American renal transplant recipients (RTR) require higher tacrolimus doses to achieve target levels compared to white RTR: does clotrimazole help? Transplant Proc. 2013;45(10):3498–501.
Lalan S, Abdel-Rahman S, Gaedigk A, Leeder JS, Warady BA, Dai H, et al. Effect of CYP3A5 genotype, steroids, and azoles on tacrolimus in a pediatric renal transplant population. Pediatr Nephrol. 2014;29(10):2039–49.
Moody DE, Liu F, Fang WB. Azole antifungal inhibition of buprenorphine, methadone and oxycodone in vitro metabolism. J Anal Toxicol. 2015;39(5):374–86.
Mordvinov VA, Shilov AG, Pakharukova MY. Anthelmintic activity of cytochrome P450 inhibitors miconazole and clotrimazole: in vitro effect on the liver fluke Opisthorchis felineus. Int J Antimicrob Agents. 2017;50(1):97–100.
Saad A, DePestel D, Carver P. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26(12):1730–44.
Viesselmann CW, Descourouez JL, Jorgenson MR, Radke NA, Odorico JS. Clinically significant drug interaction between clotrimazole and tacrolimus in pancreas transplant recipients and associated risk of allograft rejection. Pharmacotherapy. 2016;36(3):335–41.
Nakatani T, Fukushima N, Ono M, Saiki Y, Matsuda H, Nunoda S, et al. The registry report of heart transplantation in Japan (1999–2014). Circ J. 2016;80(1):44–50.
Kim JH, Han N, Kim MG, Yun HY, Lee S, Bae E, et al. Increased exposure of tacrolimus by co-administered mycophenolate mofetil: population pharmacokinetic analysis in healthy volunteers. Sci Rep. 2018;8(1):1687.
Undre NA, Schafer A. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation. European Tacrolimus Multicentre Renal Study Group. Transpl Proc. 1998;30(4):1261–3.
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53.
Shi WL, Tang HL, Zhai SD. Effects of the CYP3A4*1B Genetic Polymorphism on the Pharmacokinetics of Tacrolimus in Adult Renal Transplant Recipients: a Meta-Analysis. PLoS One. 2015;10(6):e0127995.
Lamba JK, Lin YS, Thummel K, Daly A, Watkins PB, Strom S, et al. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12(2):121–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding was used to conduct this study.
Disclosure of potential conflicts of interest
Takaya Uno, Kyoichi Wada, Sachi Matsuda, Yuka Terada, Akira Oita, Atsushi Kawase, and Mitsutaka Takada declare that they have no conflicts of interest.
Ethical approval
The study protocol was approved by the National Cerebral and Cardiovascular Center Ethics Committee (reference number M27-067-5).
Informed consent
Written informed consent regarding the disclosure of clinical data was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uno, T., Wada, K., Matsuda, S. et al. Impact of the CYP3A5*1 Allele on the Pharmacokinetics of Tacrolimus in Japanese Heart Transplant Patients. Eur J Drug Metab Pharmacokinet 43, 665–673 (2018). https://doi.org/10.1007/s13318-018-0478-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0478-6