Abstract
Background and objectives
Schizophrenia and Alzheimer’s disease are characterised by glutamatergic pathway abnormalities related to N-methyl-d-aspartate (NMDA) receptor hypofunction and cognitive impairment. Glycine is an NMDA receptor co-agonist; inhibition of glycine transporter 1 (GlyT1) should improve NMDA receptor hypofunction. This study evaluated safety and pharmacokinetic properties of BI 425809—a potent and selective GlyT1 inhibitor.
Methods
In the single-rising dose (SRD) component of this study, subjects were randomised to a single dose of BI 425809 [doses (mg): 0.5, 1, 2, 5, 10, 25, 50, 100 and 150], or placebo. The bioavailability/food effect (BA/FE) component investigated BI 425809 pharmacokinetics following single dosing (25-mg tablet) after overnight fasting or with a high-calorie meal or as solution (25 mg) after overnight fasting.
Results
Overall, 33/83 (39.8%) subjects had ≥ 1 treatment-related adverse event (AE); there were no deaths or serious AEs. Reported SRD part AEs trended towards dose dependency, occurring at the higher doses (mostly central nervous system related). BI 425809 plasma concentration–time profiles were similarly shaped across all doses and plasma exposure increased proportional to dose. In the BA/FE component, geometric mean ratios for the area under the concentration–time curve from time zero to the last measurable concentration and the maximum plasma concentration for tablet fasted versus solution fasted were 80.5 and 50.0%, respectively, and for tablet fed versus fasted were 125.9 and 142.1%, respectively.
Conclusion
BI 425809 was generally well-tolerated at doses expected to be clinically relevant. The AE profile suggested possible GlyT1-inhibiting effects.
Clinical trial identifier
NCT02068690.
Similar content being viewed by others
BI 425809, a novel GlyT1 inhibitor, was well-tolerated within a dose range (0.5–25 mg) expected to be clinically relevant. |
Adverse events showed a trend towards dose-dependency. |
The pharmacokinetic profile of BI 425809 favours a once-daily dosing regimen. |
1 Introduction
Cognitive impairment is a core feature of neurodegenerative diseases such as Alzheimer’s disease (AD), and is also present in psychiatric disorders such as schizophrenia [1, 2]. Both schizophrenia and AD are characterised by abnormalities in glutamatergic pathways related to N-methyl-d-aspartate (NMDA) receptor hypofunction [2, 3]. These abnormalities have been associated with cognitive impairment in schizophrenia and AD [4], and with negative symptoms, such as social withdrawal, decreased eye contact and decreased or muted facial expression in schizophrenia [5]. The NMDA receptors are activated by glutamate and glycine, in which glycine acts as an obligatory co-agonist at the glycine modulatory site on the receptors. Inhibition of glycine transporter 1 (GlyT1; a transporter that regulates the synaptic levels of glycine), should improve NMDA receptor hypofunction by increasing the concentration of glycine in the synaptic cleft [6]. It is hypothesised that an increase in NMDA receptor signalling leads to an increase in synaptic plasticity, which should improve cognitive function and memory (Fig. 1).

Figure adapted from Moschetti et al. [17]
Proposed mechanism of action of GlyT1 inhibition on glutamatergic neurotransmission. AMPA-R α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, Ca 2+ calcium, GlyT1 glycine transporter 1, NMDA-R N-methyl-d-aspartate receptor
BI 425809, a potent and selective GlyT1 inhibitor, is a new chemical entity, hypothesised to improve cognitive function and memory in patients with schizophrenia and AD [7]. The objective of this Phase I first-in-human study was to evaluate the safety, tolerability and pharmacokinetic profile of single doses of BI 425809, compared with placebo, in healthy male volunteers, and to assess the effect of formulation or food on the pharmacokinetic profile of BI 425809.
2 Methods
2.1 Study Design
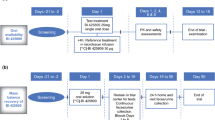
This was a Phase I, first-in-human study, conducted at a single site in Germany in healthy male volunteers (NCT02068690), which was split into two parts. The first part was a partially randomised, placebo-controlled, single-blind, single-rising dose (SRD) component. The partially randomised design was utilised to maintain, for safety reasons, an initial treatment sequence of active–placebo–active–active 1 h apart in the first cohort of each dose level, ensuring an interval of at least 2 h between the first and second active dose of each dose level, which was expected to be sufficient to detect relevant acute effects of BI 425809 whilst protecting patient safety. This part aimed to assess the safety and tolerability of single doses of BI 425809, ranging from 0.5 to 150 mg. The secondary aim was to investigate the pharmacokinetic profile of BI 425809, including dose proportionality. The second part of the trial was a randomised, open-label, three-way cross-over bioavailability/food effect (BA/FE) component involving three treatment periods. Part 2 aimed to assess the relative bioavailability of BI 425809 as a tablet or oral solution in the fasted state, and the relative bioavailability of the tablet in the fed versus fasted state.
2.1.1 Part 1: SRD Component Design
Eight volunteers were randomised to a single dose of BI 425809 as solution or placebo at each intended dose level: 0.5, 1, 2, 5, 10, 25, 50, 100 and 150 mg, administered in a fasted state. Within each dose group, six subjects received the active drug and two received placebo. For safety reasons, each dose group was split into cohorts of four subjects each. The randomisation was conducted using a computerised random number generator and was performed in blocks of four. This part of the study was conducted across 3 visits [Visit 1: screening; Visit 2: drug administration and plasma sampling (on Day 1 of each visit)], followed by continuous medical care of a physician or the medical staff for 34 h after administration and adequate safety monitoring up to Day 9 of each visit; and Visit 3: end-of-study examination].
For pharmacokinetic assessment, 2.7 mL of venous blood samples were taken at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, 34, 48, 72, 96, 120, 144, 168 and 192 h using an indwelling cannula. All blood samples were centrifuged at 2000–4000 g at 4–8 °C for at least 10 min, with intermittent storage on ice. The plasma obtained was split into two cryotubes, which were frozen immediately (no later than 60 min after blood sampling). Both aliquots contained at least 0.5 mL of plasma (one for the analysis and the other was used as an analytical back-up sample). The plasma samples were then stored frozen, in upright position at − 20 °C or below until analysis. Urine was collected 0–4, 4–8, 8–12 and 12–24 h and then at 24-h intervals up to 192 h to determine the concentration of BI 425809 excreted.
2.1.2 Part 2: BA/FE Component Design
A separate group of 12 healthy volunteers was randomised (using a computerised random number generator) to receive (1) BI 425809 25 mg as a tablet in the fasting state (reference treatment, R), (2) BI 425809 25 mg as a tablet after a standardised high-fat, high-calorie meal (test treatment, T1) and (3) BI 425809 25 mg as solution in the fasting state (test treatment, T2); subjects were thereby allocated to one of six possible treatment sequences: R/T1/T2, R/T2/T1, T1/T2/R, T1/R/T2, T2/T1/R or T2/R/T1. This part of the study was conducted across five visits [Visit 1: screening; Visits 2, 3 and 4: drug administration and plasma sampling (on Day 1 of each visit), followed by pharmacokinetic collection and safety monitoring up to Day 9 of each visit (each treatment was separated by a washout phase of at least 14 days); and Visit 5: end-of-study examination].
BI 425809 was administered after an overnight fast of at least 10 h for the fasting assessments and 30 min after a high-calorie, high-fat breakfast for the fed assessments. Blood samples for pharmacokinetic assessment were taken at the same time points as in Part 1, after treatment administration.
Both parts of the study were approved by the Independent Ethics Committee at the Human Pharmacology Centre of Boehringer Ingelheim Pharma GmbH and Co. KG, Ingelheim, Germany, according to national and international regulations. The study was conducted in accordance with the International Conference for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice and local legislation, in accordance with the principles of the Declaration of Helsinki [10, 11]. All subjects provided written, informed consent before procedures were performed.
2.2 Treatments
2.2.1 Part 1: SRD Treatments
BI 425809 was provided as a prepared solution in amber glass bottles at concentrations of 0.5, 5 or 50 mg/mL. Solutions were prepared at the study site before administration using BI 425809 powder for reconstitution and a solvent of 55% polyethylene glycol 400, 30% vitamin E polyethylene glycol succinate, 10% ethanol and 5% purified water (Boehringer Ingelheim Pharma GmbH & Co. KG, Germany). Placebo solutions contained only the solvent. Subjects were blinded to treatment.
2.2.2 Part 2: BA Treatments
Subjects received BI 425809 25 mg as a 5-mg/mL solution or as a 25-mg tablet.
2.3 Subjects
2.3.1 Inclusion Criteria
Subjects included in this study were healthy male volunteers, 18–45 years of age, with a body mass index (BMI) of 18.5–29.9 kg/m2.
2.3.2 Exclusion Criteria
Subjects were excluded if they showed evidence of a concomitant disease upon examination, vital sign assessment, electrocardiograms (ECG) or laboratory test [follicle-stimulating hormone (FSH), luteinising hormone (LH) and haemoglobin values were to be strictly within the reference range]. Other exclusion criteria included gastrointestinal, hepatic, renal, respiratory, cardiovascular, metabolic, immunological or hormonal disorders; diseases of the central nervous system (such as epilepsy), other neurological disorders or psychiatric disorders; history of relevant orthostatic hypotension, fainting spells or blackouts; chronic or relevant acute infections; history of relevant allergy/hypersensitivity (including allergy to the trial medication or its excipients) or evidence or history of macular degeneration or any abnormal finding in colour discrimination test. Subjects were also excluded if they had a history of surgery of the gastrointestinal tract that could have interfered with kinetics of the study drug, had taken drugs with a long half-life (more than 24 h) within 30 days or less than 10 half-lives before study drug administration or had use of any drug within the previous 10 days that may have influenced the study results or prolonged QT interval. Additional exclusion criteria included drug abuse, excessive alcohol intake (> 20 g/day), use of tobacco (> 10 cigarettes, three cigars/pipes per day) or inability to refrain from smoking on study days and blood donation of > 100 mL within 30 days before the study.
2.4 Study Endpoints and Assessments
The primary endpoint was the frequency [n (%)] of subjects with drug-related adverse events (AEs) following single-rising doses, as determined by the investigator. An AE was defined as any untoward medical occurrence, including an exacerbation of a pre-existing condition, in a subject who received the study drug. The event did not have to have a causal relationship with the treatment. The intensity of AEs were graded as mild (awareness of symptom[s] which were easily tolerated), moderate (enough discomfort to cause interference with usual activity) or severe (incapacitating/causing inability to work or to perform usual activities).
AEs included clinically relevant findings from safety laboratory tests (chemistry; haematology; enzymes and hormones including LH, FSH, testosterone and sex hormone-binding protein), neurological examination, 12-lead ECGs, vital signs (blood pressure), oxygen saturation (SpO2), physical examinations (occurrence of findings), visual tests (colour discrimination, Amsler grid and visual acuity), and Bond and Lader [8] and Bowdle [9] visual analogue scales (VAS) for possible psychedelic effects. Safety assessments were carried out at several time points through Days 1–9 and spontaneous AEs (including details of the time of onset, the end time and the intensity of these events) were recorded throughout the study. Subjects were kept under close medical surveillance until at least 34 h following drug administration and were allowed to leave the trial site after formal assessment and confirmation of their fitness by the investigator.
Secondary endpoints included the pharmacokinetic parameters of maximum plasma concentration (C max) and area under the concentration–time curve from time zero extrapolated to infinity (AUC0–∞) of BI 425809. Other pharmacokinetic parameters examined were AUC from time zero to the last measurable concentration (AUC0–tz ), AUC from time interval t 1 to t 2 (AUC t1–t2), time to C max (t max), terminal half-life (t ½), fraction eliminated in urine from time point t 1 to t 2 \( (f{\text{e}}_{{t_{1} {-}t_{2} }} ) \), amount of analyte in urine over the time interval t 1–t 2 \( (A{\text{e}}_{{t_{1} {-}t_{2} }} ) \), apparent clearance after extravascular administration (CL/F) and apparent volume of distribution during the terminal phase after extravascular administration (V z/F).
2.5 Bioanalytical Assay
Concentration of BI 425809 in both plasma and urine were analysed at BI Pharmaceuticals, Inc., Ridgefield, CT, USA, using a validated liquid chromatography tandem mass spectrometry method.
The concentration of BI 425809 was determined over the range of 1.00–1000 nM using linear calibration standard curves (seven concentration levels) with 1/x 2 weighting. Drug concentration data identified below the lower limit of quantification (BLQ) were displayed as such and not replaced by zero at any time point (including the lag phase and predose values). For the non-compartmental analysis, concentration values identified as BLQ in the lag phase were set to zero. The lag phase was determined as the period between time zero and the first time point with a concentration above the quantification limit.
During the study, assay performance was assessed by back-calculation of calibration standards, tabulation of the standard curve fit function parameters and measurement of quality control samples. The quality control plasma samples were prepared in K3-ethylenediaminetetraacetic acid human plasma at three concentration levels and those for the urine samples were prepared in 0.05% Tween 20 human urine, also at three concentration levels. These samples were analysed to assess accuracy and precision of pharmacokinetic measurements.
The pharmacokinetic software used in this study was Phoenix WinNonlin Version 6.3 (for the non-compartmental analysis). Statistical analysis software, Version 9.2 was used to produce graphs and tables.
2.5.1 Criteria for Relative Bioavailability Investigation
For trials investigating relative bioavailability, the cross-over design is viewed favourable due to its efficiency: since each subject served as his own control, the comparison between the treatments was based on a comparison within subjects rather than between subjects. Thus, the inter-subject variability was removed from the comparison between treatments, resulting in a lower number of subjects required.
2.6 Statistical Analysis
2.6.1 Part 1: Determination of Sample Size
A total of 72 subjects were planned for inclusion in this component of the study. The planned sample size was not based on a power calculation; eight subjects per dose group (six on active treatment and two on placebo) is commonly used in SRD studies of this nature and is generally considered sufficient for the exploratory evaluation of single dose safety and pharmacokinetics [12].
2.6.2 Part 1: SRD Statistical Analysis
All subjects from Part 1 who received active treatment of BI 425809 and had at least one observation for the endpoints of C max, AUC0–∞, \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \) and \( A{\text{e}}_{{t_{1} {-}t_{2} }} \) without important protocol violations were included for statistical analysis. C max, AUC0–∞, \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \) and \( A{\text{e}}_{{t_{1} {-}t_{2} }} \) were assessed for dose proportionality using a power model in which a regression model was applied to log-transformed data. The corresponding analysis of covariance model included the logarithm of the dose as a covariate. Analysis of safety, disposition, demographic data and baseline characteristics included all subjects who received at least one dose of the study drug.
2.6.3 Part 2: Determination of Sample Size
A total of 12 subjects were planned for inclusion in this component of the study, with all subjects receiving BI 425809 25 mg. With this sample size, sufficient precision [defined through the ratio of upper to lower confidence interval (CI) limit] in estimating the ratio of geometric Means (gMeans; test/reference) could be expected with 95% probability.
2.6.4 Part 2: BA/FE Statistical Analysis
All subjects from Part 2 of the study with at least one observation for at least one of the endpoints of AUC0–∞, \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \) or C max of BI 425809 were included for statistical analysis. Analysis of safety, disposition, demographic data and baseline characteristics included all subjects who received at least one dose of study drug. Relative bioavailability was based on an analysis of AUC0–∞, \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \) or C max parameters, using the transformed natural logarithms. The statistical model analysed variance on the logarithmic scale, while including sequence, subjects within sequence and period and treatment effects to account for potential variation. The difference between the expected means for test treatments and reference treatment was estimated by the difference in the corresponding least-squares means (point estimate), and their two-sided 90% CIs based on the t distribution were computed. These quantities were then back transformed to the original scale to give the point estimator gMean and interval estimates for the ratio between value-under-test and value-under-reference treatments.
2.6.5 Parts 1 and 2: Safety Endpoints
Safety data were analysed descriptively.
3 Results
3.1 Study Population and Subject Disposition
A total of 83 subjects were entered into the trial, all of whom completed the trial and were included in the evaluation. In Part 1, 72 subjects were assigned to nine sequential dose groups: 54 subjects received BI 425809 and 18 subjects received placebo. Dose escalation was terminated after 150 mg (maximally tolerated dose) owing to dose-limiting AEs that were observed at this dose.
In Part 2 (BA/FE), 11 subjects were treated with BI 425809. Two subjects received the wrong treatment at Visit 3 and for this visit only they were excluded from pharmacokinetic but not from safety analyses. None of the subjects discontinued the trial early.
The demographic data for Part 1 (SRD), Part 2 (BA/FE) and both parts together are shown in Table 1. All 83 healthy volunteers were white men. Across both groups, the mean (standard deviation; SD) age of the subjects was 32.5 (7.9) years. The mean BMI (SD) was 25.5 (2.27) kg/m2. The demographic characteristics across dose groups in both components (SRD and BA/FE) were largely similar, except that subjects receiving BI 425809 10 mg were younger (mean 25.0 years) than in the other SRD groups (range of means 29.3–38.5 years).
3.2 Safety and Tolerability
During both parts of the study, 47/83 subjects (56.6%) reported at least one AE (Table 2). Overall, 33 subjects (39.8%) had at least one AE that the investigator assessed as related to treatment: 27 (37.5%) in Part 1 and six (54.5%) in Part 2 (see below for further details). There were no clinically relevant changes in laboratory parameters, including hormone levels [FSH, LH, sex hormone-binding protein and testosterone (total, free, free androgen index)], haematology or vital signs (including SpO2) seen in either part of the study. No clinically relevant abnormal findings for ECGs were reported. Individual ECG intervals were mostly within or only slightly outside the respective reference ranges with a few exceptions. In general, no statistically relevant differences in changes from baseline of QTcF interval, QT interval or heart rate (HR) between placebo and any of the doses tested in Part 1, and no indications of a dose–response relationship were found. No clinically relevant abnormal findings for neurological examinations were reported. There were no serious AEs reported.
In the SRD group, 39/72 subjects (54.2%)—34/54 receiving BI 425809 (63.0%) and 5/18 receiving placebo (27.8%)—experienced at least one AE. There was a dose-related increase in the incidence of AEs in this part of the study, from 16.7% in the 0.5-mg group to 100% in the 150-mg group. Except for one, all AEs were of mild or moderate intensity. One severe AE occurred after administration of BI 425809 150 mg: vomiting accompanied by nausea, vertigo and a moderate headache as well as blurred vision and mild dizziness. This AE was considered drug related and required treatment. The subject recovered from all events on the same day of the drug administration.
The most commonly reported AEs in Part 1 were headache, fatigue, blurred vision, vertigo, dizziness, somnolence, photopsia and back pain. In terms of nervous system disorders, headache was more common at lower doses (0.5–25 mg) whereas dizziness, somnolence and ataxia predominated at higher doses. Eye disorders appeared to be dose related and were only seen at doses of 100 or 150 mg; in the 100-mg group, one subject experienced blurred vision and, in the 150-mg group, all six subjects reported at least one of the following AEs: photopsia (flashing lights), blurred vision or chromatopsia (change in colour perception). Colour discrimination and visual acuity tests showed no changes from baseline, although two subjects in total, receiving BI 425809 25 and 100 mg, reported abnormalities on the Amsler grid test. These abnormalities were recorded as ‘visual field tests abnormal’ and ‘vision blurred’, respectively. Bond and Lader VAS and Bowdle VAS scores for subjective feelings of sedation showed an increase at doses of 10 mg and greater. Bond–Lader VAS scores of other items (calmness, contentment and aspects of external and internal perception) were increased at the dose levels of 100 and 150 mg.
It is worth mentioning, that the frequently reported AEs of nervous system and eye disorders reported in the higher dose groups (drowsiness, vertigo, tiredness, ataxia, blurred vision, photopsia) occurred shortly after administration (usually within 1 h) and resolved within 2–3 h. Headache was reported at different time points on the first day of dosing and generally disappeared within 12 h.
3.3 Pharmacokinetic Results
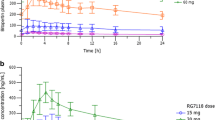
3.3.1 Part 1: SRD Pharmacokinetics
The plasma concentration–time profiles of BI 425809 were similarly shaped at all dose levels assessed and were characterised by rapid absorption (median t max of ~ 45 min) followed by at least a biphasic distribution phase (Fig. 2a). Inter-individual variability (as shown by the geometric coefficient of variation) ranged from 8 to 49% for pharmacokinetic parameters based on plasma levels and from 24.8 to 95.2% for parameters based on urine levels. The t ½, CL/F, V Z/F and fractional excretion in urine were independent of administered dose (Table 3). The slope of the linear regression analysis was close to one for plasma pharmacokinetic exposure (C max, AUC0–∞ and \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \)) and urinary excretion (Ae0–192), indicating a high degree of dose proportionality across the dose range tested (Table 4).
3.3.2 Part 2: BA/FE Pharmacokinetics
The median t max in plasma was 0.875 h for the 25-mg solution under fasted conditions and increased to 4 h for the 25-mg tablet under fasted conditions. When the 25-mg tablet was administered under fed conditions, the median t max was 1.77 h (Fig. 2b). Thus, the plasma pharmacokinetic profiles were shaped differently between the treatment groups. The absorption phase was longer under fasted conditions, resulting in a later t max, but the distribution and the elimination phases were similarly shaped across all treatments. The bioavailability of BI 425809 administered as a tablet was higher under fed conditions than in the fasted state (Table 5).
The bioavailability of BI 425809 administered as a tablet was lower than the bioavailability of BI 425809 administered as oral solution (Table 5). The adjusted gMean ratios for \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \) and C max for comparison of BI 425809 administered as a tablet fasted versus oral solution fasted were 80.5% (90% CI 74.0, 87.6%) and 50.0% (90% CI 45.1, 55.4%), respectively. The adjusted gMean ratios for \( {\text{AUC}}_{{0{-}t_{\text{z}} }} \) and C max for comparison of BI 425809 tablet administered under fed versus fasted conditions were 125.9% (90% CI 115.7, 137.0%) and 142.1% (90% CI 128.3, 157.4%), respectively.
4 Discussion
This study demonstrated that a single dose of BI 425809 was generally well-tolerated at the anticipated therapeutic dose range of 5–25 mg, with increased incidences of AEs at higher doses. A qualitative review of the frequency of AEs showed relatively few AEs; those that were observed, including drowsiness, vertigo, tiredness, ataxia, blurred vision, photopsia, were generally in line with reports of other GlyT1 inhibitors [13,14,15]. Similar to recently published studies, incidences of AEs increased dose dependently [13]. The most common AEs were central nervous system and visual effects. In this study, the incidences of nervous system disorders (fatigue, dizziness, somnolence and ataxia), eye disorders (photopsia, blurred vision and chromatopsia) and ear and labyrinth disorders (vertigo) occurred with increased frequency and intensity at the dose levels 100 and 150 mg. Further dose escalation was, therefore, stopped at 150 mg. The occurrence of the nervous system and eye disorders appeared around 1 h after drug administration, and therefore, correlates with the rapid absorption of BI 425809 (t max values of approximately 45 min after drug administration) and disappear with declining plasma concentration of BI 425809. A signal for a potential sedating effect and perceptual alterations was observed at higher doses of BI 425809. There were no significant changes in hormone levels after a single oral dose of BI 425809 in the current study. These observations provide important information regarding safety profile, which will support the ongoing clinical development of BI 425809.
The concentration–time profiles of BI 425809 were similar across all dose groups and dose proportionality was observed in all exposure parameters (\( {\text{AUC}}_{{0{-}t_{\text{z}} }} \), AUC0–∞, C max) across the entire dose range (0.5–150 mg). Fractional renal excretion was low (5%) and similar across all dose groups. The gMean t ½ of BI 425809 was not dose related and ranged between 32.5 h and 47.0 h. Overall, the kinetics of BI 425809 reinforces the suitability of the BI 425809 tablet for once-daily administration.
The bioavailability of BI 425809 from the 25-mg tablet was lower than from the 25-mg solution. There was also a difference in the median t max due to different absorption phases, but the curves from the distribution and elimination phases were similarly shaped. The bioavailability of BI 425809 from a 25-mg tablet was higher after food than when taken in a fasted state. Consequently, the AE profile of doses observed in this study may be somewhat different from that observed of similar doses with the tablet formulation, which has lower exposure. Both observations might be explained by the physiochemical properties of BI 425809, as it is a Class II compound (according to the Biopharmaceutics Classification System) [16] with low intrinsic solubility and high permeability. The administration of BI 425809 with food may increase its intrinsic solubility.
A limitation of Part 1 of this study is that the safety profile may not be predictive for higher dose levels of the tablet as it is not only the total exposure level but also the absorption kinetics that could trigger AEs. Additionally, as with typical first-in-human studies, there could be no conclusions on drug behaviour in women, elderly volunteers and patients. Also, there could be no conclusions on drug behaviour after multiple doses of BI 425809 or possible pharmacodynamic effects, as both were not investigated. Finally, because of the small number of subjects in this study, rare AEs could not be identified.
5 Conclusions
In this first-in-human single-dose study, BI 425809 was well-tolerated by healthy male subjects within a clinically relevant dose range (up to 25 mg) but higher doses showed increased incidences of AEs and the occurrence of sedative effects and perceptual alterations. The pattern of AEs was in line with a possible GlyT1-inhibiting effect. The pharmacokinetic profile of BI 425809 reinforces the suitability of the BI 425809 tablet for once-daily administration.
References
Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150(1):42–50. doi:10.1016/j.schres.2013.07.009.
Hu N-W, Ondrejcak T, Rowan MJ. Glutamate receptors in preclinical research on Alzheimer’s disease: update on recent advances. Pharmacol Biochem Behav. 2012;100(4):855–62. doi:10.1016/j.pbb.2011.04.013.
Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol Biochem Behav. 2012;100(4):665–77. doi:10.1016/j.pbb.2011.03.023.
Lakhan SE, Caro M, Hadzimichalis N. NMDA receptor activity in neuropsychiatric disorders. Front Psychiatry. 2013;4:52. doi:10.3389/fpsyt.2013.00052.
Rubio MD, Drummond JB, Meador-Woodruff JH. Glutamate receptor abnormalities in schizophrenia: implications for innovative treatments. Biomol Ther. 2012;20(1):1–18. doi:10.4062/biomolther.2012.20.1.001.
Hashimoto K. Glycine transport inhibitors for the treatment of schizophrenia. Open Med Chem J. 2010;4:10–9. doi:10.2174/1874104501004010010.
Rosenbrock H, Giovannini R, Schmid B, Kramer G, Arban R, Dorner-Ciossek C, et al. Improving cognitive function in rodents via increasing glycine levels in brain by the novel glycine transporter-1 inhibitor BI 425809. Alzheimer’s Dement J Alzheimer’s Assoc. 2016;12(7):P1018. doi:10.1016/j.jalz.2016.06.2099.
Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211–8. doi:10.1111/j.2044-8341.1974.tb02285.x.
Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88(1):82–8. doi:10.1097/00000542-199801000-00015.
ICH Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, ICH harmonised tripartite guideline: Guideline for Good Clinical Practice, E6(R1). 1996.
The World Medical Association. Declaration of Helsinki—ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-4. doi:10.1001/jama.2013.281053.
Broom C. Design of first-administration studies in healthy man. Early phase drug evaluation in man. London: Macmillan Press; 1990. p. 206–13.
Hirayasu Y, Sato S-I, Takahashi H, Iida S, Shuto N, Yoshida S, et al. A double-blind randomized study assessing safety and efficacy following one-year adjunctive treatment with bitopertin, a glycine reuptake inhibitor, in Japanese patients with schizophrenia. BMC Psychiatry. 2016;16:66. doi:10.1186/s12888-016-0778-9.
D’Souza DC, Singh N, Elander J, Carbuto M, Pittman B, de Haes JU, et al. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology. 2012;37(4):1036–46. doi:10.1038/npp.2011.295.
Ouellet D, Sutherland S, Wang T, Griffini P, Murthy V. First-time-in-human study with GSK1018921, a selective GlyT1 inhibitor: relationship between exposure and dizziness. Clin Pharmacol Ther. 2011;90(4):597–604. doi:10.1038/clpt.2011.154.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. doi:10.1023/A:1016212804288.
Moschetti V, Boland K, Feifel U, Hoch A, Zimdahl-Gelling H, Sand M. First-in-human study assessing safety, tolerability and pharmacokinetics of BI 409306, a selective phosphodiesterase 9A inhibitor, in healthy males. Br J Clin Pharmacol. 2016;82(5):1315–24. doi:10.1111/bcp.13060.
Acknowledgements
We gratefully acknowledge the contribution of Solen Pichereau from Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work presented here, including the conduct of the study, data analysis and interpretation, was funded by Boehringer Ingelheim. The sponsor was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Editorial support in the form of initial preparation of the outline based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, assembling tables and figures, copyediting and referencing was provided by Lisa Auker, PhD, of Fishawack Communications, and was funded by Boehringer Ingelheim.
Conflicts of interest
The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors. Viktoria Moschetti, Michael Desch, Sophia Goetz, Karl-Heinz Liesenfeld, Holger Rosenbrock, Klaus-Peter Kammerer, Glen Wunderlich and Sven Wind are employees of Boehringer Ingelheim, but received no direct compensation related to the development of this manuscript.
Ethics approval
All procedures in this study were in accordance with the 1964 Helsinki declaration (and its amendments), and were approved by the Independent Ethics Committee at the Human Pharmacology Centre of Boehringer Ingelheim Pharma GmbH and Co. KG, Ingelheim, Germany. All subjects provided written, informed consent before procedures were performed.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Moschetti, V., Desch, M., Goetz, S. et al. Safety, Tolerability and Pharmacokinetics of Oral BI 425809, a Glycine Transporter 1 Inhibitor, in Healthy Male Volunteers: A Partially Randomised, Single-Blind, Placebo-Controlled, First-in-Human Study. Eur J Drug Metab Pharmacokinet 43, 239–249 (2018). https://doi.org/10.1007/s13318-017-0440-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0440-z