Abstract
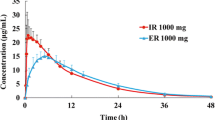
The intravenous (iv) formulation of levetiracetam has been available in clinical practice worldwide for several years, but not in Japan. Two open-label studies were conducted: Study A evaluated the bioequivalence of iv and oral tablet formulations in healthy Japanese volunteers; and Study B subsequently compared the pharmacokinetics of iv levetiracetam in healthy Japanese and Caucasian volunteers. Study A had a randomised, two-way crossover design; a single 1,500 mg levetiracetam dose was administered as a 15-min iv infusion and as 3 × 500 mg oral tablets to Japanese volunteers. In Study B, 1,500 mg levetiracetam was administered as single and repeated 15-min iv infusions to Japanese and Caucasian volunteers. Overall, 26/27 volunteers completed Study A and 32/32 (16 Japanese; 16 Caucasian) completed Study B. In Study A, the point estimate and 90 % confidence interval (CI) for the geometric least squares mean (LSM) ratio (iv vs oral) were fully included within the acceptance range for bioequivalence (0.85–1.25) for the area under plasma concentration–time curve from 0 to last quantifiable observation (AUClast 0.97 [0.95, 0.99]), but not for the maximum plasma concentration (C max 1.64 [1.47, 1.83]). In Study B, after a single iv infusion, the point estimates (90 % CI) for the geometric LSM ratio (Japanese vs Caucasian) for body weight-normalised C max and AUClast were 1.21 (1.07, 1.36) and 0.97 (0.90, 1.04), respectively. Corresponding values after repeated iv infusions were C max,ss 1.01 (0.91, 1.12) and AUCτ,ss 0.89 (0.83, 0.96). Levetiracetam was well tolerated in both studies. Study A did not demonstrate the bioequivalence of single doses of levetiracetam 1,500 mg administered as an iv infusion and as oral tablets in healthy Japanese adults. Study B, however, showed that pharmacokinetic profiles were generally similar between Japanese and Caucasian adults after single and repeated iv infusions of levetiracetam 1,500 mg.


Similar content being viewed by others
References
Baulac M, Brodie MJ, Elger CE et al (2007) Levetiracetam intravenous infusion as an alternative to oral dosing in patients with partial-onset seizures. Epilepsia 48:589–592
French J, Edrich P, Cramer JA (2001) A systematic review of the safety profile of levetiracetam: a new antiepileptic drug. Epilepsy Res 47:77–90
Gidal BE, Baltès E, Otoul C, Perucca E (2005) Effect of levetiracetam on the pharmacokinetics of adjunctive antiepileptic drugs: a pooled analysis of data from randomized clinical trials. Epilepsy Res 64:1–11
Harden C (2001) Safety profile of levetiracetam. Epilepsia 42(Suppl 4):36–39
Inoue T, Suzuki A, Yoshida K, Yamamoto K (2014) An open-label, multicenter study to evaluate the safety of adjunctive treatment with intravenous infusion of levetiracetam in adult epilepsy patients with partial seizures [Japanese with English abstract]. Jpn J Clin Psychopharmacol 17:413–422
Patsalos PN (2004) Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet 43:707–724
Perucca E, Gidal BE, Baltès E (2003) Effects of antiepileptic comedication on levetiracetam pharmacokinetics: a pooled analysis of data from randomized adjunctive therapy trials. Epilepsy Res 53:47–56
Pigeolet E, Jacqmin P, Sargentini-Maier ML, Stockis A (2007) Population pharmacokinetics of levetiracetam in Japanese and Western adults. Clin Pharmacokinet 46:503–512
Ramael S, Daoust A, Otoul C et al (2006a) Levetiracetam intravenous infusion: a randomized, placebo-controlled safety and pharmacokinetic study. Epilepsia 47:1128–1135
Ramael S, de Smedt F, Toublanc N et al (2006b) Single-dose bioavailability of levetiracetam intravenous infusion relative to oral tablets and multiple-dose pharmacokinetics and tolerability of levetiracetam intravenous infusion compared with placebo in healthy subjects. ClinTher 28:734–744
Shah VP, Midha KK, Dighe S et al (1991) Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet 16:249–255
Toublanc N, Lacroix D, Yamamoto J (2014) Development of an integrated population pharmacokinetic model for oral levetiracetam in populations of various ages and ethnicities. Drug Metab Pharmacokinet 29:61–68
Weinstock A, Ruiz M, Gerard D et al (2013) Prospective open-label, single-arm, multicenter, safety, tolerability, and pharmacokinetic studies of intravenous levetiracetam in children with Epilepsy. J Child Neurol 28:1423–1429
Acknowledgments
This study was sponsored by UCB Pharma. UCB Pharma was involved in the design and conduct of the study, and collection, management and analysis of the data. The authors thank Azita Tofighy, Ph.D. (UCB Pharma) for review and coordination of manuscript preparation, Mieko Shirakawa (Medical Co. LTA Sumida Hospital, Tokyo, Japan) for clinical pharmacy support and critical review of the manuscript and Dr. Steven Warrington (Hammersmith Medicines Research, London, UK) for critical review of the manuscript. Ioana Edgeworth, Ph.D. and Jennifer Stewart, M.Sc. (QXV Communications, Macclesfield, UK) provided writing support, funded by UCB Pharma.
Conflict of interest
Nathalie Toublanc, Takuya Okagaki, Robert Chan, Shikiko Watanabe, Katsumi Yamamoto, Katsumi Yoshida and Jens-Otto Andreas are employed by UCB Pharma. Malcolm Boyce and Ayumi Mugitani have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toublanc, N., Okagaki, T., Boyce, M. et al. Pharmacokinetics of the antiepileptic drug levetiracetam in healthy Japanese and Caucasian volunteers following intravenous administration. Eur J Drug Metab Pharmacokinet 40, 461–469 (2015). https://doi.org/10.1007/s13318-014-0227-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-014-0227-4