Abstract
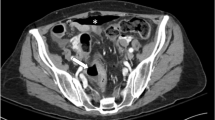
Bevacizumab is largely used in colorectal cancer patients. Postsurgical healing complications have been described for patients following treatment with Bevacizumab. We report three cases of spontaneous intestinal perforation following infusion of Bevacizumab. From January 2002 through October 2010, Bevacizumab was delivered in 143 patients. Spontaneous intestinal perforation occurred in 3 cases (2.1 %). Bevacizumab may result in severe complications. Therefore, it is important to consider every patient treated with Bevacizumab at risk for life-threatening gastro-intestinal complications.
Similar content being viewed by others
References
Carmelit P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69:4–10
Thornton AD, Ravn P, Winslet M et al (2006) Angiogenesis inhibition with bevacizumab and the surgical management of colorectal cancer. Br J Surg 93:1456–1463
Verhoef C, de Wilt JH, Verheul HM (2006) Angiogenesis inhibitors: perspectives for medical, surgical and radiation oncology. Curr Pharm Des 12:2623–2630
Hurwitz H, Kabbinavar F (2005) Bevacizumab combined with standard fuoropyrimidine-based chemotherapy regimens to treat colorectal cancer. Oncology 69:17–24
Saif MW, Elfiky A, Salem RR (2007) Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 14:1860–1869
Kabbinavar F, Hurwitz HI, Fehrenbacher L et al (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Giantonio B, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results From the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Gordon CR, Rojavin Y, Patel M, James EZ, Grana G, Kann B et al (2009) A review on bevacizumab wound healing. Ann Plast Surg 62:707–709
Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D, First BEAT Investigators (2009) Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 20(11):1842–1847
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3:391–400
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342
Sanjaykuma H, David C, Shenhong Wu (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 10(6):559–568
Begè T, Lelong B, Iret F, Tuttini O, Guiramand J, Topart D et al (2009) Bevacizumab-related surgical site complication despite primary tumor resection in colorectal cancer patients. Ann Surg Oncol 16(4):856–860
Lordick F, Geinitz H, Theisen J, Sendler A, Sarba M (2006) Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. Int. J. Radiat Oncol Biol. Phys. 64:1295–1298
August DA, Serrano D, Poplin E (2008) “Spontaneous”, delayed colon and rectal anastomotic complications associated with bevacizumab therapy. J Surg Oncol 87:180–185
Acknowledgments
The authors declare that they have no conflict of interest.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borzomati, D., Nappo, G., Valeri, S. et al. Infusion of Bevacizumab increases the risk of intestinal perforation: results on a series of 143 patients consecutively treated. Updates Surg 65, 121–124 (2013). https://doi.org/10.1007/s13304-013-0207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-013-0207-2