Abstract
Introduction
Many patients with diabetes may require high-dose insulin treatment to achieve target HbA1c level, but the prevalence, disease burden, and patient characteristics of the population remain unclear. We therefore investigated people with insulin-treated diabetes in the UK from 2009 to 2013, who were prescribed high daily doses (> 200 units/day).
Methods
A retrospective analysis was conducted using the UK primary care electronic dataset from the Clinical Practice Research Datalink (CPRD). Trends of demographics, insulin dose, clinical characteristics, and annualized incidence rate of the insulin initiators were analyzed. Patients with type 1 (T1D) or type 2 diabetes (T2D) and who were prescribed insulin between 2009 and 2013 were categorized into either a low- or high-dose insulin user group. Two-sample t test and chi-square test were used for comparison of continuous variables and categorical variables, respectively. A multivariable negative binomial regression analysis with (log) person time as an offset was used to assess the impact of different covariates on incidence of high-dose insulin initiation.
Results
Between 2009 and 2013, 19,631 patients with diabetes were treated with insulin (T1DM, 7620; T2DM, 12,011). In 2013, 415 high-dose insulin initiators were identified (T1DM, N = 170; T2DM, N = 245). More than half were male (T1DM/T2DM, 62.4%/56.3%) and 94.1%/83.7% of T1DM/T2DM patients were prescribed an insulin analogue at high-dose insulin initiation. At 6 months, 43.6% of T1DM and 42.6% of T2DM remained to have suboptimal HbA1c level of ≥ 8% (64 mmol/mol). Overall, 15.9%/63.3% of high-dose insulin initiators (HDII) with T1DM/T2DM took oral antidiabetics. From 2009 to 2013, the estimated glomerular filtration rate worsened in both T1DM and T2DM HDII.
Conclusion
Despite a number of patients requiring high doses of insulin in the UK, achievement of optimal HbA1c levels remains poor. Early identification of HDII is important in order to plan for alternative/adjuvant antidiabetic and lifestyle strategies to achieve optimal glycemic targets in this patient group.
Funding
Eli Lilly and Company.
Similar content being viewed by others
Introduction
In the UK, the number of people diagnosed with diabetes increased 2.5-fold between 1996 and 2014. Estimates suggest that about 1.1 million people have diabetes but are undiagnosed. Data from the Health Survey for England and estimates by Diabetes UK report that approximately 5 million people across the UK have impaired glucose regulation [HbA1c between 42 and 46 mmol/mol (6.0–6.4%)] [1]. Currently, the National Health Service (NHS) spends about 10% of its budget on diabetes with over three-quarters of it being utilized to treat the complications of diabetes [1, 2].
There is extensive evidence that tight glucose control reduces the risk of long-term complications of diabetes (DCCT [3], UKPDS [4]). Accordingly, national (National Institute for Health and Care Excellence) [5, 6] and international guidelines [American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) statement] [7] have recommended the need to intensify treatment to achieve optimal HbA1c levels. A previous study [8] derived from a UK electronic primary care database reported that the crude prevalence rate of insulin use has increased from 2.43 (95% CI 2.38–2.49) per 1000 population in 1991 to 6.71 (6.64–6.77) per 1000 in 2010, especially among people with type 2 diabetes (0.67–4.34 per 1000 population). Despite current guidelines, a focused review of literature by Khunti and Millar-Jones, in 2017, highlighted clinical inertia with insulin intensification in patients with type 2 diabetes as a more pronounced chronic problem in the UK than the rest of the world [9].
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [10] reported increased mortality with intensive glucose control therapy versus standard therapy; but a subanalysis of the study by Riddle [11] found that the excess mortality occurred in the intensive arm, in which the patients would have received intensive escalation of insulin therapy but were not able to achieve HbA1c lowering.
Therefore, earlier identification of patients who would require high doses of insulin will allow physicians to implement more focused treatment approaches. Unfortunately, information on the extent of high-dose insulin usage in the UK is limited. Therefore, the aim of this study was to describe the patient characteristics, disease burden, and trends of insulin utilization, especially high-dose insulin (> 200 units/day) per year from 2009 to 2013.
Methods
Data Sources
This study was a retrospective analysis of UK general practice data from the Clinical Practice Research Datalink (CPRD). The CPRD service is a representative of the UK population providing anonymized longitudinal electronic primary care records for public health research since 1987. It collects coded data including but not limited to demographics, prescribing and diagnostic information, referrals, results, pregnancies, vaccinations, lifestyle characteristics, and covers therapy areas such as heart disease, cancer, mental health, diabetes, asthma, and women’s health. It contains data of over 11.3 million patients from 674 practices with a median follow-up of 5.1 years [12, 13]. We extracted data on the trend of patients’ demographics, insulin initiation dose, and clinical characteristics from year 2009 to 2013.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Study Population
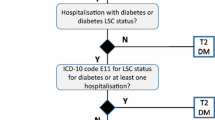
Patients diagnosed with either type 1 or type 2 diabetes and who used insulin every year between 2009 and 2013 were identified and grouped into two categories groups based on the amount of insulin total daily dose (TDD) [14]; the low-dose group comprised patients prescribed a TDD of ≤ 200 units/day; high-dose insulin users (HDIU) were patients receiving a TDD of > 200 units/day. Another category was high-dose insulin initiators (HDII) in each year comprising patients who had any TDD > 200 units/day during that year but a TDD of ≤ 200 units/day in the previous 6 months. All medical conditions were identified on the basis of recorded READ codes [15, 16], providing the standard clinical vocabulary by which clinicians record patient findings and procedures in health and social care IT systems across primary and secondary care in the UK.
Patients were excluded if they had a specific diagnosis of gestational diabetes, secondary diabetes mellitus, or polycystic ovary syndrome, were prescribed only metformin, did not have diabetes codes, or were registered with the practice for less than 3 months during the study.
Definition of Key Measurements and Main Outcomes
Total daily dose was defined as a 3-month average daily prescribed dose, calculated by dividing the total insulin prescribed during each quarter by the number of days in each quarter.
Prevalence of High-Dose Insulin Users
The T1DM and T2DM study cohorts were further divided into two categories based on the amount of TDD of insulin prescribed: (1) low-dose insulin user (LDIU) group or (2) high-dose insulin user (HDIU) group.
For analysis of prevalence of HDIU, we included as our numerator all individuals who had been prescribed high-dose insulin treatment within that year. The denominator included all individuals using insulin in that year. For an HDIU in each year, the index date was defined as the first high-dose insulin prescription date.
Incidence of High-Dose Insulin Initiation
For each year from 2009 to 2013, if a patient had TDD > 200 units/day during that year and TDD ≤ 200 units/day in the previous 6 months, the person was identified as an HDII for that year. The index date was defined as the date when the first high dose of insulin was prescribed with no prescription of high-dose insulin in the preceding 6 months.
For our analysis on incidence of high-dose insulin initiation, annualized incidence rate (AIR) of HDII was estimated per 1000 person-years at risk (PYAR), calculated by totaling the number of patients with the first use of high-dose insulin each year, and dividing this number by the total person-years of follow-up for all patient records for that year. Person time (in years) was measured from the latest of the registration date, up-to-standard (date at which data in the practice is considered to have continuous high quality data fit for use in research) date and January 1 of that year to the earliest of high-dose insulin initiation date, left the practice (transfer out) date, practice last collection date, date of death, and end of that year.
Statistical Analyses
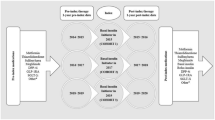
All analyses were conducted separately for people with T1DM and T2DM. In 2013, for both the LDIU and HDIU groups, baseline (6 months prior to index date) patient demographics, clinical characteristics (e.g., BMI, duration of diabetes, and lipid measurements), and concomitant medication use were calculated and compared. Furthermore, for HDII, the antidiabetic treatment (insulin, oral medications, GLP-1 receptor agonist (GLP-1 RA) use, and the ratio of basal to bolus dosage), and disease burden (HbA1c measurements and e-GFR) were collected and compared between 6 months pre- and post-index period.
For the aforementioned analyses, two-sample t test and chi-square test were used for comparison of continuous variables (e.g., age) and categorical variables (e.g., gender), respectively.
Medication utilization was also presented to describe the changes over time in the prescribing patterns in primary care. The percentage of patients (either HDIU or HDII) prescribed different antidiabetic therapies (e.g., insulin regimen, oral antidiabetic drugs (OADs), or GLP-1RA) and nondiabetic medications (e.g., antihypertension, antidyslipidemia medications, etc.,) was determined for the years 2009, 2011, and 2013.
To assess the impact of different covariates, multivariable negative binomial regression analysis with (log) person time as an offset was used to analyze changes in incidence by age, gender, socioeconomic status, clinical characteristics, and medication utilization in a calendar year while controlling for the other respective variables. Likelihood ratio tests were used to explore the significance of each variable in the univariate and final adjusted multivariable model. This was done using the data from 2009, 2011, and 2013 as well.
SAS (Version 9.2) was used to conduct all the aforementioned analyses.
Results
In total, 19,631 (7620 and 12,011 with T1DM and T2DM, respectively) patients on insulin treatment for diabetes between 2009 and 2013 (Fig. 1) satisfied the selection criteria. During the 5-year timeframe, the average weight and BMI of insulin users with T1DM decreased slightly but remained relatively stable for those with T2DM (Fig. 2). An increasing number of patients required high doses of insulin to control hyperglycemia (143–247 and 332–443 HDIU with T1DM and T2DM, respectively); however, the prevalence rate of HDIU decreased, especially for those with T2DM (6.7–5.2%) (Fig. 1).
Baseline Characteristics of HDIU
The population characteristics for HDIU are summarized in Table 1. In 2013, 247 and 443 HDIU were identified among patients with T1DM and T2DM, respectively. Patients with T2DM were older than those with T1DM. More than half of the patients were male in both groups, with HDIU having a higher percentage of males than the LDIU group.
The mean weight and BMI of patients were both higher in the HDIU vs LDIU, and were highest in the T2DM HDIU. All patients had comparable HbA1c values. The proportion of patients with HbA1c ≥ 9% in the LDIU/HDIU groups was 40.4%/38.7% for T1DM and 43.0%/41.9% for T2DM, respectively. As for Charlson Comorbidity Index (CCI) score, the LDIU and HDIU groups were quite similar in both diabetes types.
Treatment Outcomes of HDII in 2013
A total of 415 HDII (170 and 245 with T1DM and T2DM, respectively) were identified (Table 2). In the 6 months after high-dose insulin initiation, despite a slight decrease in average HbA1c (8.6% vs 9.1% with P < 0.05) for HDII with T2DM, more than half of the patients continued to have HbA1c ≥ 8% in both diabetes types, although the proportion was relatively lower compared to that in the 6 months prior to high-dose insulin initiation. The proportion of patients with T2DM with HbA1c ≥ 9% was significantly lower for 6 months’ post- vs pre-index. Insulin analogue was the most prevalent insulin regimen at both pre- and post-index for T1DM as well as T2DM. The ratio of basal to bolus dosage was approximately 40/60 for both the overall insulin and insulin analogue treatments among HDII with T1DM. Metformin was the most prescribed OAD in both groups. The eGFR decreased significantly in T2DM (70.2 vs 67.0, P value < 0.05) (Table 2).
Trend of Characteristics of HDII
The annualized incidence rates (AIR) of high-dose insulin initiation showed different trends in people with T1DM and T2DM (Table 3 shows the results in 2009, 2011, and 2013). For insulin users with T1DM, the AIR (in the unit of per 1000 person-years) initially increased from 2009 (28.2) to 2010 (32.4), then decreased in 2011 (27.2), and kept increasing until 2013 (31.6). For HDII with T2DM, the trend was more obvious, with a continuous decrease from 2009 to 2013 (40.4–31.3, in per 1000 person-years). Average weight and BMI decreased in HDII with T1DM (Fig. 3). However, no obvious trend was observed in weight and BMI for HDII with T2DM between 2009 and 2013.
Figure 4 shows the trend of utilization of non-insulin antidiabetic medications (OAD or GLP-1RAs), antihypertensive, antiplatelet, and lipid-lowering drugs in HDII patients and also the LDIU, both for 6 months prior to index date. For patients with T1DM, the proportion of HDIIs using OAD decreased from 22.1% in 2009 to 14.1% in 2013, and also decreased slightly from 13.9% in 2009 to 11.4% in 2013 in the LDIU. However, insulin users with T2DM showed almost no change for OAD uptake in HDII while it increased in the low-dose group from 65.9% to 69.8%. Over the same period, utilization of GLP-1 increased in people with T2DM from 2.6% to 14.7% in HDII, while there was no noticeable change for HDII patients with T1DM. From 2009 to 2013, the proportions also increased for the LDIU from 0.2% to 0.5% in patients with T1DM and from 1.6% to 7.9% in patients with T2DM. Throughout the period of study, proportion of HDII with T2DM using antihypertensive, antiplatelet, and lipid-lowering drugs was higher than that of HDII patients with T1DM. The trend was similar for low-dose groups with T2DM versus T1DM. For HDII in 2013, the insulin TDD was 3.3 units/kg for T1DM and 2.6 units/kg for T2DM while the weight was 84.2 kg for T1DM and 102.5 kg for T2DM.
Factors Associated with High-Dose Insulin Initiation
Table 4 shows factors associated with incidence of HDII. For insulin users with T1DM, higher BMI was associated with higher incidence of HDII, and higher incidence was seen in the year 2013 compared to 2009. Women had a lower incidence of HDII than men. For insulin users with T2DM, relative risk of HDII increased with BMI, duration of diabetes, antihypertensive and antiplatelet treatments, and decreased in 2013 as compared to 2009.
Discussion
From 2009 to 2013, average weight and BMI decreased in HDII with T1DM (BMI 31.2–29 kg/m2, weight 90.1–84.2 kg). This trend was similar to data published on a group of obese patients with T2DM in a study by Paul et al. [17]. The reason for this is unclear but poor compliance may play a role in the observed weight loss. For HDII in 2013, in the 6 months following high-dose insulin initiation, a high proportion of HDII patients had HbA1c ≥ 8%; 56.4% in T1DM and 57.4% in T2DM. This result has important implications, as in the post hoc analysis of the ACCORD study [10], a higher mortality rate was experienced by patients who were inadequately controlled at baseline and received intensive glucose-lowering strategy but still had suboptimal glucose control (HbA1c > 7%; > 53 mmol/mol). An increase in insulin resistance may be the driver of this poor outcome and may also lead to the increasing dose because BMI trends did not show an increase in our study. Indeed, the characteristics of patients in the HDII cohort are of those with metabolic syndrome manifested by increased BMI, systolic blood pressure, and raised triglyceride levels.
Although the number of people living with diabetes in the UK has increased almost 2.5-fold since 1996, the percentage of HDIU has decreased. This may be explained by the reported higher prevalence of clinical inertia with insulin intensification and availability of other insulin-sparing agents in the UK than rest of the world [9]. Clinical inertia to insulin intensification is the reluctance of many patients and clinicians to initiate and intensify insulin therapy in spite of the fact that early and strict glycemic control following diagnosis of diabetes is key to achieving positive clinical outcomes. The review reported that the reasons for reluctance might primarily arise from lack of clinical expertise, time, and patient understanding. Use of other glucose-lowering therapies (concomitant medications) to control HbA1c has decreased nonsignificantly for patients with T2DM. However, HbA1c control remained suboptimal for a variety of reasons, which include insulin resistance [18], longer diabetes duration, poor compliance to insulins [19], and obesity [20]. Given the rising prevalence of obesity in the general population with diabetes, persistent high HbA1c values, and the nature of patients receiving HDII, increased use of novel glucose-lowering therapies which target the insulin resistance and metabolic syndrome states is important.
This study had several limitations. The CPRD database collects data from medical practices in the UK only. Therefore, the generalization of results to other countries needs to be evaluated. There may have been a delay in entering patients’ information into the system, which may have resulted in bias in determining the calendar year time point. The observational nature of the retrospective design could have introduced selection bias for evaluating the longitudinal change of prevalence/incidence. All medical conditions were identified on the basis of the READ codes recorded in the CPRD database without comparisons with medical charts or laboratory results. The TDD is an estimation based on 3 months’ data of insulin prescribed, but not the amount that was actually taken. The retrospective methodology does not allow for observing the manner in which the individual patient may have self-adjusted the insulin dosing. Actual daily doses prescribed by the health care professional (HCP) are not very well captured in CPRD (e.g., this may be captured in patient notes and only for a few patients). Finally, we were not able to adjust human behavior such as patient motivation, compliance, and adverse effects of high dose insulin, which is not collected by the CPRD database. Additional research needs to be performed to provide insights into clinical inertia and fill the gap in our study.
Conclusion
From 2009 to 2013, an increasing number of patients required high doses of insulin to control hyperglycemia. Although increasing BMI was associated with increasing high-dose insulin initiation, the average BMI among insulin users did not increase from 2009 to 2013. In addition, achievement of optimal HbA1c levels remains poor. High concomitant non-insulin antidiabetic medication use, poor glycemic control in the follow-up year, and HCP inertia suggest that alternative intensive insulin therapy may be considered for this challenging population. Early identification of HDII is important in order to plan for alternative/adjuvant antidiabetic and lifestyle strategies to achieve optimal glycemic targets in this patient group. Further research is needed to examine changes in real-world clinical long-term outcomes after high-dose insulin initiation.
References
https://www.diabetes.org.uk/Documents/Position%20statements/DiabetesUK_Facts_Stats_Oct16.pdf. Accessed 31 July 2018.
http://onlinelibrary.wiley.com/doi/10.1111/j.1464-5491.2012.03698.x/epdf. Accessed 31 July 2018.
Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. https://doi.org/10.2337/dc13-2112.
Genuth S, Eastman R, Kahn R, et al. Implications of the United Kingdom prospective diabetes study. Diabetes Care. 2003;26(suppl 1):s28–32.
NICE guideline NG 17. Type 1 diabetes in adults: diagnosis and management. 26 August 2015. https://www.nice.org.uk/guidance/ng17/resources/type-1-diabetes-in-adults-diagnosis-and-management-1837276469701. Accessed 12 Feb 2018.
NICE guideline NG 28. Type 2 diabetes in adults: management. 2 December 2015. https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493. Accessed 12 Feb 2018.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
Holden SE, Gale EA, Jenkins-Jones S, Currie CJ. How many people inject insulin? UK estimates from 1991 to 2010. Diabetes Obes Metab. 2014;16(6):553–9.
Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122(8):844–6.
CPRD. Clinical Practice Research Datalink. 2017. https://www.cprd.com/home/. Accessed 10 June 2017.
Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–36.
Vincent V. Medicines management team wirral adapted from: Pan Mersey statement on high strength insulin products and WUTH risk minimisation strategy. NHS. http://mm.wirral.nhs.uk/document_uploads/guidelines/high-strength-insulin-strategy-updated-January17.pdf. Accessed 31 July 2018.
UK Read Code. 2017. https://data.gov.uk/dataset/uk-read-code. Accessed 10 June 2017.
Booth N. What are read codes? Health Libr Rev. 1994;11(3):177–82.
Paul SK, Shaw JE, Montvida O, Klein K. Weight gain in insulin-treated patients by body mass index category at treatment initiation: new evidence from real-world data in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(12):1244–52.
Kamuhabwa AR, Charles E. Predictors of poor glycemic control in type 2 diabetic patients attending public hospitals in Dar es Salaam. Drug Healthc Patient Saf. 2014;6:155–65.
Shuvankar M, Biswanath S, Kaushik KD, Agnihotri B, Deb A. Compliance to anti-diabetic drugs: observations from the diabetic clinic of a medical college in Kolkata, India. J Clin Diagn Res. 2013;7(4):661–5.
Yousefzadeh G, Shokoohi M, Najafipour H. Inadequate control of diabetes and metabolic indices among diabetic patients: a population based study from the Kerman Coronary Artery Disease Risk Study (KERCADRS). Int J Health Policy Manag. 2014;4(5):271–7.
Acknowledgements
Funding
This study and the article processing charges were supported by Eli Lilly and Company, Indianapolis, USA. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Xiaomei Peng is an employee and stockholder of Eli Lilly and Company and/or one of its subsidiaries. Xuanyao He is an employee and stockholder of Eli Lilly and Company and/or one of its subsidiaries. Dongju Liu is an employee and stockholder of Eli Lilly and Company and/or one of its subsidiaries. Elemer Balogh is an employee and stockholder of Eli Lilly and Company and/or one of its subsidiaries. Puneet Kaushik is an employee and stockholder of Eli Lilly and Company and/or one of its subsidiaries. Kate von Brunt was an employee of Eli Lilly and Company at the time of this study. Iskandar Idris has nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This is an observational research program and does not impose any form of intervention on the investigator. Hence, the assessment and treatment of the subject was based solely on the investigator’s routine or usual practice in the provision of care to subjects with diabetes. The subject provided authorization for the uses and disclosures of their personal health information as described in the study Consent to Release Information. This consent covers the collection and release of data regarding treatment and its outcomes for the entire period of the study. The confidential nature of the subject information was maintained. The study was reviewed by CPRD’s Independent Scientific Assessment Committee (CPRD Protocol Approval Number: 15_075).
Data Availability
All data generated or analyzed during this study are included in this published article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7088537.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Idris, I., Peng, X., He, X. et al. The Trend of High-Dose Insulin Usage Among Patients with Diabetes in the UK: A Retrospective Study. Diabetes Ther 9, 2245–2257 (2018). https://doi.org/10.1007/s13300-018-0515-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0515-0