Abstract
Introduction
Based on existing data regarding the durability of liraglutide in type 2 diabetes, this study aimed to assess its long-term effectiveness at 5 years and its overall impact on cardiovascular (CV) risk.
Methods
This was a multicenter retrospective observational study. Liraglutide was used under routine clinical practice conditions. Changes from baseline to 60 months in HbA1c, fasting plasma glucose (FPG), body weight, blood pressure, and lipid profile were assessed. United Kingdom Prospective Diabetes Study (UKPDS) scores were calculated at baseline and after 60 months to assess changes in the estimated 5- and 10-year risk for fatal and nonfatal coronary heart disease (CHD) and fatal and nonfatal stroke.
Results
Overall, 103 patients (age 59.0 ± 7.9 years, diabetes duration 10.4 ± 6.8 years) were involved in the study. After 60 months, HbA1c levels were reduced by − 1.0 ± 1.2%, FPG levels by − 24.5 ± 43.4 mg/dl, body weight by − 5.3 ± 6.4 kg, systolic blood pressure by − 6.5 ± 18.5 mmHg, diastolic blood pressure by − 3.6 ± 11.8 mmHg, and total cholesterol by − 16.9 ± 37.4 mg/dl. The proportion of patients achieving HbA1c levels of < 7% increased from 12.7% to 39.8% (p = 0.02). Based on the UKPDS scores, statistically significant reductions in the 5- and 10-year risk of nonfatal CHD and fatal CHD were found, with no change in the 5- and 10-year risk of fatal and nonfatal stroke.
Conclusion
In patients prolonging treatment with liraglutide for 5 years, the benefits in relation to metabolic control and CV risk factors are maintained. The UKPDS risk scores suggest that liraglutide is associated with a reduced CHD risk, but not with a reduced stroke risk.
Similar content being viewed by others
Introduction
Liraglutide is a recombinant analogue of native human GLP-1 used as therapeutic option in type 2 diabetes (T2DM). Liraglutide has shown, in addition to glycemic effects, a well-documented series of actions on extraglycemic systems that are critical to diabetes therapy [1, 2].
In various studies, the use of liraglutide alone or in combination with other oral hypoglycemic agents (OHAs) was found to be associated with statistically significant reductions in HbA1c, ranging from − 0.6% to − 1.6%, and in fasting plasma glucose (FPG), ranging from − 13 to − 43 mg/dl. Liraglutide was also associated with a significant reduction in body weight, ranging from − 1.0 to − 3.2 kg, depending on the dose administered. Observed reductions in systolic blood pressure (SBP) range from − 0.6 to − 6.7 mmHg [2,3,4,5,6,7]. Reductions in SBP are observed early, after just 2 weeks of treatment with liraglutide, irrespective of weight loss [8]. In addition, the lipid profile was found to improve in patients treated with liraglutide [2, 9].
Given its impact on these main markers of cardiovascular (CV) risk in T2DM, liraglutide can clearly help to reduce CV risk in T2DM patients [10, 11].
These assumptions were recently confirmed by the LEADER™ cardiovascular outcomes trial [12], which demonstrated the CV safety profile of liraglutide used in combination with oral antidiabetic drugs and/or insulin. Compared to placebo, liraglutide significantly reduced (by 13%) the incidence of the primary endpoint (major adverse cardiac events—MACE: CV death, nonfatal myocardial infarction, nonfatal stroke).
All available studies of liraglutide—both randomized clinical trials (RCTs) and real-world evidence (RWE) research—consistently show improvements in metabolic control and CV risk factors with the use of this drug, and underline the reproducibility of relevant experimental data in the real world [13, 14]. However, none of those studies included more than 3.5 years of follow-up data.
Based on existing data regarding the durability of the drug, the aim of our study was to assess if liraglutide maintains its long-term effectiveness in relation to metabolic control and CV risk factors even after 5 years of treatment.
Methods
This was a multicenter retrospective observational study carried out at ten sites in the Veneto and Trentino Alto Adige regions of Northeast Italy. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients before they were included in the study.
Inclusion criteria were:
-
Previous diagnosis of T2DM
-
Initiation of treatment with liraglutide due to inadequate metabolic control (HbA1c ≥ 7.5% or 53 mmol/mol) during the calendar year of 2011, with therapy maintained until at least September 2016
-
Male and female patients
-
At least one annual follow-up visit in the 60 months following therapy initiation
The exclusion criteria for liraglutide therapy initiation were:
-
Acute or infectious disease
-
Established or suspected neoplastic disease
-
Clinical history of acute pancreatitis
-
Pregnancy (also suspected) or breastfeeding
-
Generic contraindications to treatment with liraglutide
The following baseline parameters were collected: age, sex, weight, body mass index (BMI), systolic and diastolic blood pressure, lipid profile (total cholesterol, HDL cholesterol, and triglycerides), duration of diabetes, FPG, HbA1c, creatinine, and glucose-lowering therapies.
In accordance with the European technical data sheet for liraglutide and the indications of the Italian Medicines Agency (AIFA), liraglutide was prescribed at an initial dose of 0.6 mg/day, which was subsequently increased to 1.2 mg/day after the first 7 days of treatment, and then possibly to 1.8 mg/day in the case of inadequate glycemic control.
The follow-up data at 60 months after baseline considered in the analysis were: HbA1c, FPG, weight, BMI, blood pressure, and lipid profile.
All of the data were extracted directly by the investigators from the electronic clinical records of the individual sites and added to a unified database. All consecutive patients who started liraglutide during 2011 were included in the study to minimize the selection bias.
Statistical Analysis
Values of continuous variables are expressed below as the mean ± standard deviation (SD), and values of categorical variables are presented as frequencies (%).
Baseline and follow-up information was used to calculate the UKPDS scores [15, 16], which were employed to assess the changes in the 5- and 10-year risk for fatal and nonfatal coronary heart disease (CHD) and fatal and nonfatal stroke.
Longitudinal comparisons in terms of metabolic control, blood pressure, lipid profile, body weight, and UKPDS scores were performed using the paired Student’s t test or the Wilcoxon signed-rank test, respectively, for normal and non-normal variables. Analyses were performed on the overall sample and stratified by the change in UKPDS score for the 10-year risk of nonfatal CHD (i.e., any improvement from baseline to 60 months vs. no change or worsening).
p < 0.05 was considered to indicate a statistically significant difference. The software SPSS ver. 20 was used to carry out all of the statistical tests.
Results
Overall, 103 patients were involved in the study. Baseline characteristics are reported in Table 1. The mean age was 59.0 (± 7.9) years, 52.4% were men, and the mean duration of diabetes was 10.4 (± 6.8) years.
After 5 years of treatment with liraglutide, statistically significant improvements were obtained in the following clinical outcomes: HbA1c level was reduced by − 1.0 ± 1.2%, FPG level by − 24.5 ± 43.4 mg/dl, body weight by − 5.3 ± 6.4 kg, SBP by − 6.5 ± 18.5 mmHg, DBP by − 3.6 ± 11.8 mmHg, and total cholesterol by − 16.9 ± 37.4 mg/dl. The proportion of the patients achieving HbA1c levels of < 7% increased from 12.7% to 39.8% (p = 0.02) (Table 2 and the Electronic supplementary material).
Concomitant medications associated with liraglutide at the start of treatment were as follows: 93.1% of patients were treated with metformin, 47.1% with a sulfonylurea, 30.4% with basal insulin, and 6.9% with a thiazolidinedione. In addition, 18.6% of patients switched from a dipeptidyl peptidase-4 inhibitor and 4.9% from another GLP-1 receptor agonist. After 5 years of treatment with liraglutide, 96% of patients were treated with metformin, 43% with a sulfonylurea, 22% with basal insulin, and 7% with a thiazolidinedione. Treatment regimens at baseline and at 60 months are shown in Fig. 1.
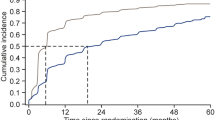
After 1 year of treatment, 59% of patients were treated with a liraglutide dose of 1.2 mg/day and 35% of patients with a dose of 1.8 mg/day. After 5 years of treatment, 83% of patients were treated with 1.8 mg/day, 16% with 1.2 mg/day, and 1% of patients with 0.6 mg/day.
UKPDS scores at baseline and after 60 months of treatment with liraglutide are shown in Table 3. Statistically significant reductions in the 5- and 10-year risk of nonfatal CHD and fatal CHD were found, while a neutral effect on the 5- and 10-year risk of fatal and nonfatal stroke was documented.
Regarding the factors that could positively or negatively impact CHD and stroke risk, it was noted that, compared with patients who did not show a benefit of liraglutide according to their UKPDS scores, patients who had a reduction in the 10-year risk of nonfatal CHD (n = 49) had a greater reduction in HbA1c (− 1.3 ± 1.3% vs. − 0.64 ± 0.99%; p = 0.005), a greater reduction in body weight (− 6.7 ± 6.7 kg vs. − 3.9 ± 5.8 kg; p = 0.028), a greater reduction in total cholesterol (− 34.8 ± 37.5 mg/dl vs. − 0.63 ± 31.1 mg/dl; p < 0.001), and a greater reduction in systolic blood pressure (− 13.8 ± 18.1 mmHg vs. + 0.1 ± 16.4 mmHg; p < 0.001).
Discussion
This study has demonstrated the 5-year effectiveness of liraglutide in T2DM and confirmed the drug’s durability in patients who respond positively to therapy. Statistically significant and clinically relevant improvements in metabolic control, obesity indices, blood pressure, and lipid profile with liraglutide have previously been documented. Similar results were also obtained in this cohort without the need to substantially intensify the glucose-lowering therapy. Indeed, the proportion of patients who were being treated with insulin in association with liraglutide (with or without other OHAs) had decreased by over 10% after 5 years of liraglutide treatment. Furthermore, 83% were being treated with the maximum dose of liraglutide (1.8 mg) after 5 years of liraglutide therapy.
Reductions in the 5- and 10-year risk of fatal and nonfatal CHD were documented, while no impact on the 5- and 10-year risk of fatal and nonfatal stroke was observed.
The improvements in clinical outcomes obtained in this study are consistent with experimental and observational studies on liraglutide [9,10,11,12,13,14,15,16,17,18,19,20], and it is known that RCTs and observational studies can be used synergistically to obtain evidence of the efficacy and safety of drugs [21]. Recently, preliminary Italian data obtained 36 months after starting liraglutide have been published [9, 14]; these data show that significant improvements in glycemic control and body weight as well as additional benefits in relation to cardiovascular risk are still maintained 3 years after starting liraglutide treatment. In the LEADER study, the follow-up period was 3.5 years [12]; to the best of our knowledge, the present study of liraglutide is the first to include 5 years of follow-up. Before this study, there were no data on the impact of liraglutide on UKPDS scores, so a comparison of the UKPDS scores in the present work with corresponding data in a previous study is not possible. However, in line with the LEADER study, the results of our study suggest that cardiovascular protection is plausible and can be achieved through improved control of risk factors included in the UKPDS risk engine. In addition, there is increasing preclinical evidence of a positive action of liraglutide on the CV system, endothelial function, and the expression of mediators of systemic inflammation [11, 22]. Further studies are needed to clarify the mechanisms underlying the cardiovascular role of the drug. Based on UKPDS scores for fatal and nonfatal stroke, no benefits of liraglutide were documented. We hypothesize that the main risk factors for stroke in type 2 diabetes are age, hypertension, and the presence of atrial fibrillation [23]. Other evidence suggests that the improved glycemic balance achieved with liraglutide has a positive effect on the risk of CHD but not on the risk of stroke [24].
Our study has strengths and limitations. The main strengths of the present analysis are the long follow-up period and multicenter design, which allow it to provide important information on the long-term effectiveness of liraglutide. As for limitations, the study has a retrospective design and was based on the collection of data already recorded in electronic medical records. As such, the completeness of the information was influenced by the attitude of each center towards recording clinical data in a standardized format suitable for statistical analyses. Furthermore, as it is focused on assessing the effectiveness of liraglutide over 5 years, the study lacks a control group. Nevertheless, given the observational nature of the study, the selection of any control group would have been prone to selection bias. Finally, the results of our study can only be applied to patients who show a positive response to liraglutide, which would permit the prolongation of the treatment for 5 years.
Conclusions
In this real-life retrospective observation of 103 patients with T2DM who received liraglutide under routine clinical practice conditions, the durability of the effect of this drug on metabolic control and CV risk factors was documented after 5 years of treatment. All of these benefits translate into reductions in the 5- and 10-year risk of fatal and nonfatal CHD, as summarized by UKPDS risk score changes, but not into risk reductions for fatal and nonfatal stroke.
References
Drucker D, Nauck M. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–701.
Lapolla A, Berra C, Boemi M, Bossi AC, Candido R, et al. Long-term effectiveness of liraglutide for treatment of type 2 diabetes in a real-life setting: a 24-month, multicenter, non-interventional, retrospective study. Adv Ther. 2018;35:243–53.
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81.
Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S, LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268–78.
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR, LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32:84–90.
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simó R, Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met_SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55.
Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L, LEAD-4 Study Investigators. Efficacy and safety of the human GLP-1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met_TZD). Diabetes Care. 2009;32:1224–30 (Erratum in Diabetes Care 2010;33:692).
Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014;28:399–405.
Rondinelli M, Rossi A, Gandolfi A, Saponaro F, Bucciarelli L, Adda G, Molinari C, Montefusco L, Specchia C, Chiara Rossi M, Scardapane M, Arosio M, Genovese S. Use of liraglutide in the real world and impact at 36 months on metabolic control, weight, lipid profile, blood pressure, heart rate, and renal function. Clin Ther. 2017;39:159–69.
Sivertsen J, Rosenmeier J, Holst JJ, Vilsbøll T. The effect of glucagon-likepeptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9:209–22.
Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Ostawal A, Mocevic E, Kragh N, Xu W. Clinical effectiveness of liraglutide in type 2 diabetes treatment in the real-world setting: a systematic literature review. Diabetes Ther. 2016;7:411–38.
Ponzani P, Scardapane M, Nicolucci A, Rossi MC. Effectiveness and safety of liraglutide after three years of treatment. Miner Endocrinol. 2016;41:35–42.
Stevens RJ, Kothari V, Adler AI, Stratton IM, United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101:671–9.
Zinman B, Schmidt WE, Moses A, Lund N, Gough S. Achieving a clinically relevant composite outcome of an HbA1c of < 7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012;14:77–82.
Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;:672658.
Lapolla A, Frison V, Bettio M, Dal Pos M, Rocchini P, Panebianco G, Tadiotto F, Da Tos V, D’Ambrosio M, Marangoni A, Ferrari M, Pianta A, Balzano S, Confortin L, Lamonica M, Marin N, Strazzabosco M, Brun E, Mesturino CA, Simoncini M, Zen F, Bax G, Bonsembiante B, Cardone C, Dal Frà MG, Gallo A, Masin M, Piarulli F, Sartore G, Simioni N. Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real-world setting in Italy. Clin Ther. 2015;37:574–84.
Berkovic MC, Bilic-Curcic I, Herman Mahecic D, Gradiser M, Grgurevic M, Bozek T. Long-term effectiveness of liraglutide in association with patients’ baseline characteristics in real-life setting in Croatia: an observational, retrospective, multicenter study. Diabetes Ther. 2017;8:1297–308.
Martinez L, Penfornis A, Gautier JF, Eschwège E, Charpentier G, Bouzidi A, Gourdy P. Effectiveness and persistence of liraglutide treatment among patients with type 2 diabetes treated in primary care and specialist settings: a subgroup analysis from the EVIDENCE study, a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther. 2017;34:674–85.
Hannah EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008;1:211–7.
Courrèges JP, Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Verhoeven R, Bugáñová I, Madsbad S. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129–31.
Davis TM, Millns H, Stratton IM, Holman RR, Turner RC. Risk factors for stroke in type 2 diabetes mellitus; United Kingdom Prospective Diabetes Study (UKPDS) 29. Arch Int Med. 1999;159:1097.
Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72.
Acknowledgements
Funding
This was a nonprofit investigators-initiated initiative. The agency Airon Communication supported the clinicians with editorial assistance. This assistance included medical writing services (provided by CORESEARCH—http://www.coresearch.it) and article processing charges. No funding or sponsorship was received for the study itself. These services were funded through an unconditional grant from Novo Nordisk S.p.A. The authors of the publication are fully responsible for its contents and conclusions. Novo Nordisk S.p.A. did not influence and has not been involved in the data interpretation and statistical analysis presented in the manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Medical writing support was provided by Giuseppe Prosperini of CORESEARCH. This was funded using an unconditional grant from Novo Nordisk S.p.A.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
The following authors declare no conflict of interest or financial implications: Vera Frison, Natalino Simioni, Alberto Marangoni, Sara Balzano, Carmela Vinci, Luciano Zenari, Lorena De Moliner, Federica Tadiotto, Michele D’Ambrosio, Loris Confortin, Narciso Marin, Simonetta Lombardi, Silvana Costa, Giuseppe Prosperini, Annunziata Lapolla.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients before they were included in the study.
Data Availability
The datasets analyzed during the current study are available from the authors on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7029323.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Frison, V., Simioni, N., Marangoni, A. et al. Clinical Impact of 5 Years of Liraglutide Treatment on Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus in a Real-Life Setting in Italy: An Observational Study. Diabetes Ther 9, 2201–2208 (2018). https://doi.org/10.1007/s13300-018-0503-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0503-4