Abstract
Introduction
To explore the accumulated evidence concerning the effect of intensive blood pressure control on the incidence and progression of diabetic retinopathy (DR), proliferative diabetic retinopathy (PDR) and macular edema (ME).
Methods
A number of electronic databases were searched including PubMed, EMBASE, CINAHL, Cochrane Library, conferences and proceedings. Randomized controlled trials comparing intensive blood pressure targets with conventional blood pressure targets in patients with type 2 diabetes were included. The definition of intensive versus conventional blood pressure targets was from the pertinent original studies. Meta-analyses and trial sequential analyses of randomized trials were analyzed in STATA.
Results
Eight trials randomizing 6989 patients were assessed and reviewed in full text; 3749 vs. 3240 were in each arm (intensive vs. conventional). All trials had a low risk of bias. Intensive blood pressure control supported a 17% reduction in the incidence of DR (relative risk 0.83, 95% confidence interval 0.72–0.95). Trial sequential analyses confirmed that sufficient evidence indicated a relative risk reduction above 17% for the incidence of DR when intensive blood pressure control was targeted. Heterogeneity was absent (I2 = 0%; P = 0.56). No statistically significant effect was found for intensive blood pressure targeting on the progress of DR (relative risk 0.94, 95% confidence interval 0.81–1.08). TSA showed that insufficient evidence had been found, although the Z value line appeared to have a tendency of approaching the futility boundaries. There were also no statistically significant effects on the incidence of PDR and ME (TSA-adjusted CI 0.84–1.12).
Conclusion
Intensive blood pressure control reduced the relative risk of incidence of DR by 17%. The available data were insufficient to prove or refute a relative risk reduction for the progression of DR or incidence of PDR and ME at a magnitude of 15%.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is the primary cause of visual impairment and blindness for diabetic individuals from 30 to 70 years [1]. The current standard care for preventing or delaying DR consists of strict glycemic control, while vision loss may still appear even up to the glucose control standard [2, 3]. The increased incidence of retinopathy suggests that more precautionary measures are imperative to prevent the development of the condition and subsequent blindness.
Intensive blood pressure control prevents cardiovascular events [4, 5]. Blood vessels are directly visible in the retina. Hypertension may lead to retinal macroaneurysm, retinal vascular occlusion and ischemic optic neuropathy, which further cause visual loss. Hypertension may also exacerbate the vision-threatening effects of DR.
Given the effect of blood pressure on the pathologic changes of diabetic retinopathy [6], tight blood pressure control may be another method to prevent or delay the risk of DR. Findings of previous studies indicate that intensive blood pressure targets in diabetic individuals could decrease the development and progression of diabetic retinopathy [7, 8], but results of some studies did not support the beneficial effect of intensive blood pressure targets [9,10,11]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study Group has shown that intensive blood pressure control had no beneficial effect on reducing the rate of DR in subjects with type 2 diabetes. Tight blood glucose targets in the UKPDS decreased still did not eliminate the risk of DR [12].
Given this knowledge gap, we did a meta-analysis to assess the possible effect of lowering blood pressure on diabetic retinopathy, a trial sequential analysis (TSA) to examine the changes over time and whether more studies need to be performed, by adjusting the significance levels for sparse data and multiple testing on accumulating trials. The primary aim of our study was to explore the effect of interventions to control or reduce blood pressure on the incidence and progression of DR and the incidence of proliferative diabetic retinopathy (PDR) or macular edema (ME).
Methods
Our study was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors. Therefore, ethical approval was not necessary.
Search Strategy and Selection Criteria
MEDLINE, EMBASE, and the Cochrane Controlled Trials Register were searched for articles from inception to April. 2018 using a search strategy as follows: [diabetic retinopathy, proliferative diabetic retinopathy (PDR), macular edema, diabetic maculopathy, retinal disorders, retinal disease, diabetic eye disease, or vision loss], (randomized, random, placebo-controlled, double-blind), (hypertension or blood pressure) and (angiotensin II type 1 receptor blockers, adrenergic alpha antagonist, adrenergic beta antagonists, diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, antihypertensive agents). Reference lists of identified trials and relevant reviews were also searched. To avoid missing any relevant studies in the search, reference lists of key articles were also searched for relevant articles that could have been missed. There were no publication form restrictions. Only articles in the English language were searched.
Randomized controlled trials (RCTs) investigating the effect of strict blood pressure targeting on the incidence, progression of DR, or incidence of PDR and ME were included for analysis. Two authors independently reviewed all identified abstracts and excluded clearly irrelevant hits. Characteristics of trials for analyses were extracted by two authors independently from the included reports. Although intensive versus conventional blood pressure targets were not defined uniformly in the relevant studies, the definition according to the criteria used in the original trials was taken into consideration in this meta-analysis. A summary of the included individuals’ data is presented in Table 1 [9, 13,14,15,16,17,18,19]. The included trials were divided into those with a low risk of bias and those with a high risk of bias according to the Cochrane Handbook risk of bias tool on the basis of assessment of sequence generation, blinding and concealment of allocation [20]. When all three domains were judged to have a low risk of bias, the trial was classified as having a low risk of bias. Eight trials had a low risk of bias. Articles were excluded if they were non-randomized trials, were crossover trials, or used dual therapies or quasi-experiments.
Outcomes
The definition of incidence of DR is to have mild-to-severe non-proliferative DR or proliferative DR, which is a score on the Early Treatment Diabetic Retinopathy Study (ETDRS) in individuals who had no DR signs at baseline. The definition of progression of DR is to a two-step or greater progression from baseline on the ETDRS final scale in individuals who had DR signs at baseline.
Statistics
We assessed the within- and between-study variation or heterogeneity by testing Cochran’s Q statistic [21, 22]. Heterogeneity was quantified with the I2 metric, which was independent of the number of studies in the systematic review [23]. The pooled OR was estimated using fixed effects (FE, Mantel and Haenszel) and random effects (RE, DerSimonian and Laird) models. When there was heterogeneity among studies, the pooled OR was estimated using the random effects model. Publication bias was assessed using the Egger’s regression test and Begg’s rank correlation test. Statistical manipulations were undertaken using STATA (version 13.0, StataCorp LP, College Station, TX, USA).
Trial Sequential Analysis
We conducted the trial sequential analysis (TSA). Conventional meta-analysis had the risks of random errors due to sparse data and repetitive testing [24]. TSA adjusted the confidence intervals if data were sparse or repeatedly analyzed as a result of multiple updates to allow firm conclusions. Trial sequential monitoring boundaries were employed to control the risks for type I and II errors and to indicate whether additional trials were needed. For the required information size, we calculated the incidence in the control group from the actual meta-analyses. The intervention was able to reduce the relative risk by 15%. TSA was conducted with the intention to maintain an overall 5% risk of a type I error and a power of 80%. Meta-analysis will be updated by adding component studies sequentially in the order of publication. The β-spending function was constructed to indicate futility of the intervention. We used TSA version 0.9 beta (http://www.ctu.dk/tsa) (Copenhagen Trial Unit, 2011) for these analyses.
Results
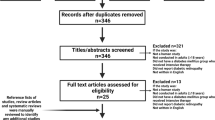
Figure 1 summarizes the results of the search. The trials included 6989 participants, of whom 3749 were randomized to intensive blood pressure control and 3240 to conventional blood pressure control [9, 13,14,15,16,17,18,19]. Table 1 shows the participants' characteristics. The range for the number of randomized patients in each trial was from 160 to 2856. All eight included trials were randomized clinical trials. The average age of individuals in each trial ranged from 55 to 66 years. Participants from three trials were either normo- or hypertensive with controlled blood pressures at baseline [9, 13, 15]. Participants from five trials had hypertension at baseline [14, 16,17,18,19].
BP and Incidence of DR
Data regarding the incidence of retinopathy were available from six of the conducted trials [9, 14, 16,17,18,19]. Compared with less or no intervention, strict blood pressure intervention supported a 17% risk of the incidence of DR (risk ratio 0.83, 95% confidence interval 0.72–0.95) (Fig. 2). Heterogeneity was absent (I2 = 0%; P = 0.56).
We did trial sequential analyses. This was similar to interim analyses in a single trial, where monitoring boundaries were used to decide whether a trial could be terminated early when a P value was sufficiently small to show the anticipated effect. Trial sequential analysis showed that the cumulative Z curve crossed the monitoring boundaries constructed from information size calculations [trial sequential analysis adjusted 95% confidence interval, 0.81 (CI 0.72–0.90) Fig. 3], thereby confirming that sufficient evidence existed for a 17% decrease in relative risk of incidence of DR when intensive blood pressure control was targeted.
Required information size to demonstrate or reject 15% relative risk reduction in the effect of strict blood pressure targets on incidence of DR with an alpha of 5% and beta of 20% is 9184 patients (vertical red line). The red dashed lines represent the trial sequential monitoring boundaries and the futility boundaries. The solid blue line is the cumulative Z curve
BP and Progression of DR
Five trials reported progression of DR that was present at the time of trial enrollment among 5132 type 2 diabetics [9, 13,14,15,16]. The overall RR of strict blood pressure for progression was 0.94 (95% CI 0.81–1.08), indicating a possible 6% reduction (Fig. 4). Heterogeneity was low (I2 = 15.6%; P = 0.31).
Trial sequential analysis showed a lack of sufficient evidence in Fig. 5. The cumulative Z curve did not cross monitoring boundaries (trial sequential analysis adjusted 95% CI 0.80–1.06). After the first three trials, the cumulative Z statistic crossed the conventional significance boundary (Z = 1.96) but did not cross the O’Brien-Fleming boundaries. From the fourth trial onwards, the meta-analysis was no longer nominally statistically significant. With the publication of the last trial, the Z score approached the futility boundaries.
Required information size to demonstrate or reject 15% relative risk reduction (a priori estimate) of the effect of strict blood pressure targets on progression of DR (with a control group proportion of 16.8%, alpha of 5%, and beta of 20%) is 7157 patients (vertical red dashed line). The red dashed lines represent the trial sequential monitoring boundaries and the futility boundaries. The solid blue line is the cumulative Z curve
BP and Incidence of PDR or Macular Edema
Data regarding the incidence of PDR or macular edema were available from five trials [13,14,15,16, 19]. No beneficial effect of blood pressure intervention was shown: RR 0.97 (95% CI 0.72–1.30), 0.93 (95% CI 0.80–1.08). Heterogeneity was present (I2 = 54.6%, P = 0.07; I2 = 15.6%; P = 0.31, for PDR or ME, respectively, Fig. 6).
Trial sequential analysis showed a lack of sufficient evidence for a 10% or greater relative risk reduction in PDR or macular edema (trial sequential analysis adjusted 95% CI 0.84–1.12). Only 6048 (28%) of the heterogeneity adjusted required information size of 21,452 patients were accrued (Fig. 7).
Required information size to demonstrate or reject 15% relative risk reduction of the effect of a strict blood pressure target on the incidence of PDR or macular edema (with a control group proportion of 13.1%, alpha of 5%, and beta of 20%) is 11,572 patients (vertical red dashed line). The red dashed lines represent the trial sequential monitoring boundaries and the futility boundaries. The solid blue line is the cumulative Z curve
Discussion
Key results of our study are (1) an intensive blood pressure target appears to be able to reduce the incidence of DR when accumulated evidence is acquired from RCTs, and the finding is subsequently confirmed by TSA. (2) RCTs fail to identify the beneficial effect of an intensive blood pressure target on the progression of DR and incidence of PDR and macular edema, while the results could not be confirmed by TSA. TSA indicated that more than 8400 individuals need to be randomized before firm decisions can be reached on any beneficial or harmful effect with a 15% RRR with a power of 80%.
The pathogenesis of diabetic retinopathy has been investigated on several biochemical pathways. The exact mechanism of hypertensive damage in DR remains unknown [25], while the hypothesis is that chronic hyperglycemia results in endothelial cell damage and breakdown of the blood-retinal barrier, which lead to dysregulation of retinal perfusion; therefore, hypertension leads to hyperperfusion damage to the eyes with DR [26,27,28]. In addition, people with DR also coincide with diabetic neuropathy; this existence of unbalance of sympathetic regulation in the retinal vessels thus could lead to the detrimental effect of hypertension on DR [25].
The cumulative Z curve crossed the monitoring boundaries constructed from both information size calculations (Fig. 3), demonstrating with 80% power that the effect of intensive blood pressure targets is > 20% relative risk reduction in the incidence of DR. Although this accumulated evidence did not identify the beneficial effect of intensive blood pressure control on the progression of DR and incidence of PDR and macular edema, the initial trial was positive at a conventional significance level of P = 0.05 (Z = 1.96), achieved by using the α-spending function and constructing the O’Brien-Fleming boundaries in TSA. In the analysis on the effect of strict blood pressure targets on the progression of DR, the cumulative Z curve approached the futility boundaries (Fig. 5), and we almost infer that the effect of intensive blood pressure targets is superior to that of conventional blood pressure control in the progression of DR, which comes with a 15% relative risk reduction.
The reasons for the discrepancy of the effect of strict blood pressure control on the incidence and progression of DR remain unknown. The discrepancy might be the different definition of the progression of DR used in the different trials. Thus, in the UKPDS, the definition of DR progression seemed crude in relation to blindness and the need for laser or vitreoretinal surgery, etc., while the progression of DR in DIRECT Protect2 was defined as an increase of three or more ETDRS levels [13]. Another possibility might be that the baseline glycated hemoglobin (HbA1c) values differed in the included various trials; nearly 11% of HbA1c in the intensive and conventional arms in the ABCD trial, while nearly 8% of HbA1c in other trials. The separate effect of blood glucose might lead to the various outcomes of diabetic retinopathy. Third, blood pressure control can sometimes be a trade-off between risks of hypotension and adequate risk factor control in people with diabetic complications, especially for people with progression of DR who could have longer duration of diabetes and more cardiovascular risk factors. The nature of the effect of blood pressure control on the progression of DR might appear to exclude the intervention of these covariants. Besides, various antihypertensive drugs were used in previous studies, and the different effects of antihypertensive drugs on diabetic retinopathy might be the reason for these conflicting findings [29]. Selection bias of language might be a limitation of this study.
In addition, our finding regarding the beneficial efficacy of intensive blood pressure targets in the development of T2D individuals with DR concords with previous observational studies, which demonstrated a detrimental effect of high blood pressure in people with DR [30], and blood pressures were only associated with new development of DR, but not with its progression [31]. Additional studies are needed to focus on why strict blood pressure targets affected the incidence but not progression of diabetic retinopathy.
The implications of our study findings for daily clinical practice should be emphasized. Understanding whether diabetic individuals have a lower risk of DR with the strict blood pressure targets will help diabetologists to provide effective clinical counseling for patients. BP optimization should be done in primary care or by a diabetologist before the patients even see the ophthalmologist.
Conclusion
Our findings show the beneficial effect of intensive blood pressure targets on the incidence of diabetic retinopathy in type 2 diabetes patients, which was confirmed by our TSA. These findings are important to healthcare practitioners, as accumulated evidence recommends that the strict blood pressure targets should be specifically tailored to these diabetic individuals with non-diabetic retinopathy. However, available evidence suggests no effect of intensive blood pressure targets on the progression of retinopathy and incidence of PDR and macular edema. Before these findings are converted into clinical practice, additional work needs to be done.
Change history
18 March 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s13300-023-01396-3
References
Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(4):209–22.
Malek M, Khamseh ME, Aghili R, Emami Z, Najafi L, Baradaran HR. Medical management of diabetic retinopathy: an overview. Arch Iran Med. 2012;15(10):635–40.
Ferris FL. How effective are treatments for diabetic retinopathy? JAMA. 1993;269(10):1290–1.
Ohashi K, Hayashi T, Watanabe Y, Hara K, Ukichi R, Asano H, Suzuki H, Yamashiro K, Tojo K, Sakamoto M, Utsunomiya K. Primary aldosteronism with type 2 diabetes mellitus requires more antihypertensive drugs for blood pressure control: a retrospective observational study. J Clin Med Res. 2018;10(1):56–62.
Ke CH, Morgan S, Smolina K, Gasevic D, Qian H, Khan NA. Is cardiovascular risk reduction therapy effective in South Asian, Chinese and other patients with diabetes? A population-based cohort study from Canada. BMJ Open. 2017;7(8):e013808.
Sjølie AK, Dodson P, Hobbs FR. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int J Clin Pract. 2011;65(2):148–53.
Jeng CJ, Hsieh YT, Yang CM, Yang CH, Lin CL, Wang IJ. Diabetic retinopathy in patients with diabetic nephropathy: development and progression. PLoS One. 2016;11(8):e0161897.
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13.
Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44.
Beulens JW, Patel A, Vingerling JR, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–36.
Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the action to control cardiovascular risk in diabetes eye study. Ophthalmology. 2014;121:2443–51.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N, DIRECT Programme Study Group. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372(9647):1385–93.
Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–63.
Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the action to control cardiovascular risk in diabetes (ACCORD) follow-on study. Diabetes Care. 2016;39(7):1089–100.
Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Matthews DR, Mogensen CE, Perkovic V, Poulter N, Rodgers A, Williams B, MacMahon S, Patel A, Woodward M, ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392–406.
Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control z in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61(3):1086–97.
Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the Delapril and Manidipine for Nephroprotection in Diabetes (DEMAND) randomized clinical trial. Hypertension. 2011;58(5):776–83.
Gaede P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of intervention 5.0.0. Cochrane Collaboration, 2008 (Available from cochrane-handbook.org).
Davey Smith G, Egger M. Meta-analyses of randomized controlled trials. Lancet. 1997;350:1182.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Crippa A, Khudyakov P, Wang M, Orsini N, Spiegelman D. A new measure of between-studies heterogeneity in meta-analysis. Stat Med. 2016;35(21):3661–75.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, GRADE Working Group. What is ‘‘quality of evidence’’ and why is it important to clinicians? BMJ. 2008;336:995–8.
Fraser-Bell S, Symes R, Vaze A. Hypertensive eye disease: a review. Clin Exp Ophthalmol. 2017;45(1):45–53.
Cardoso CRL, Leite NC, Dib E, Salles GF. Predictors of development and progression of retinopathy in patients with type 2 diabetes: importance of blood pressure parameters. Sci Rep. 2017;7(1):4867.
Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med J. 2016;22(6):589–99.
Do DV, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD006127.
Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, Wang YG. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(4):263–74.
Jin P, Peng J, Zou H, Wang W, Fu J, et al. The 5-year onset and regression of diabetic retinopathy in Chinese type 2 diabetes patients. PLoS One. 2014;9(11):e113359. https://doi.org/10.1371/journal.pone.0113359.
Cardoso CRL, Leite NC, Dib E, Salles GF. Predictors of development and progression of retinopathy in patients with type 2 diabetes: importance of blood pressure parameters. Sci Rep. 2017;7(1):4867. https://doi.org/10.1038/s41598-017-05159-6.
Acknowledgements
We thank the participants of the involved studies.
Funding
This work and article processing charges were supported by the National Science Foundation Council of China (nos. 81870556, 81670738, 81300650), Beijing Municipal Administration of Hospitals' Youth Programme (QML20170204). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Jian-Bo Zhou and Jin-Kui Yang contributed to the design of the study, analysis and interpretation of data, and prepared all figures and tables. Jian-Bo Zhou, Jin-Kui Yang, Zhi-Hui Song, Lu Bai, Xiao-Rong Zhu and Hong-Bing Li drafted a part of the manuscript. Xiao-Rong Zhu and Hong-Bing Li took part in analyzing data. All authors reviewed the manuscript.
Disclosures
The authors, Jian-Bo Zhou, Zhi-Hui Song, Lu Bai, Xiao-Rong Zhu, Hong-Bing Li and Jin-Kui Yang, have nothing to disclose.
Compliance with Ethics Guidelines
Our study was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors. Therefore, ethical approval was not necessary.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6984083.
About this article
Cite this article
Zhou, JB., Song, ZH., Bai, L. et al. RETRACTED ARTICLE: Could Intensive Blood Pressure Control Really Reduce Diabetic Retinopathy Outcomes? Evidence from Meta-Analysis and Trial Sequential Analysis from Randomized Controlled Trials. Diabetes Ther 9, 2015–2027 (2018). https://doi.org/10.1007/s13300-018-0497-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0497-y