Abstract
Introduction
With the development of new neuroimaging tools it has become possible to assess neurochemical alterations in patients experiencing chronic pain and to determine how these factors change during pharmacological treatment. The goal of this study was to examine the exact neurochemical mechanism underlying pregabalin treatment, utilizing magnetic resonance spectroscopy (1H-MRS), in a population of patients with painful diabetic polyneuropathy (PDN), with the overall aim to ultimately objectify the clinical effect of pregabalin.
Methods
A double blind, randomized, placebo-controlled study was conducted. A total of 27 patients with PDN were enrolled in the study, of whom 13 received placebo treatment (control group) and 14 received pregabalin (intervention group). Pregabalin treatment consisted of stepwise dose escalation over the study period from 75 mg daily ultimately to 600 mg daily. 1H-MRS was performed at 3T on four regions of interest in the brain: the rostral anterior cingulate cortex (rACC), left and right thalamus and prefrontal cortex. The absolute concentrations of N-acetyl aspartate, glutamate, glutamine, gamma-amino-butyric-acid (GABA), glucose (Glc) and myo-inositol (mINS) were determined using LCModel.
Results
The concentration of most neurometabolites in the placebo and pregabalin group did not significantly differ over time, with only a small significant difference in Glc level in the left thalamus (p = 0.049). Comparison of the effects of the different doses revealed significant differences for mINS in the rACC (baseline 2.42 ± 1.21 vs. 450 mg 1.58 ± 0.94; p = 0.022) and dorsolateral prefrontal cortex (75 mg 2.38 ± 0.89 vs. 450 mg 1.59 ± 0.85; p = 0.042) and also for GABA in the rACC (75 mg 0.53 ± 0.51 vs. 225 mg 0.28 ± 0.19; p = 0.014).
Conclusion
No differences were found in metabolite concentrations between the placebo (control) and intervention groups, but some differences, although small, were found between the different doses.
Trial Registration
This study is registered at ClinicalTrials.gov (NCT01180608).
Funding
Lyrica Independent Investigator Research Award (LIIRA) 2010 (Pfizer) funded the study.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a worldwide health problem of epidemic proportions, with the prevalence estimated to rise from 171 million people in 2000 to 366 million in 2030 [1]. Depending on the diagnostic criteria and population studied, the prevalence of painful diabetic polyneuropathy (PDN) varies from 8 to 26% [2,3,4]. PDN is one of the most common causes of neuropathic pain, resulting in allodynia, hyperesthesia and hyperalgesia. Patients with PDN report these symptoms mostly in the distal region of their lower extremities. Consequently, several imaging modalities, such as magnetic resonance (MR) neurography and diffusion tensor imaging, have been developed which focus on the microstructural imaging of peripheral nerves at the anatomical level of the nerve fascicles [5,6,7]. Despite these recent developments in peripheral nerve analysis, there is a consensus that chronic pain and its underlying mechanisms can be examined by brain imaging modalities, such as (functional) MR imaging (MRI) and MR spectroscopy (MRS) [8]. Such structural and functional neuroimaging studies in patients suffering from DM have shown alterations in numerous brain regions [9,10,11]. A recent meta-analysis of proton MRS (1H-nuclear MR [1H-MNR]) in DM patients indicated that several metabolite levels are altered in various regions of the brain [12]. In addition to the identification of regional metabolite alterations, 1H-NMR analysis can possibly also be used to measure treatment response and thus evaluate treatment efficacy [13]. To date, 1H-NMR has been used to identify changes in brain chemistry following the administration of pregabalin to patients diagnosed with fibromyalgia [14]. Treatment with pregabalin is, according to internationally accepted guidelines, also recommended as a first-line symptomatic treatment for PDN [15]. Prior research suggests that the analgesic effects of pregabalin may be mediated through a reduction of hyperexcitation in ascending pain pathways, with pregabalin reducing dysregulation in areas of the brain associated with pain perception and restoring the inhibitory descending nociceptive pathways [14, 16]. Several chronic pain studies utilizing 1H-MRS have shown increased glutamate (Glu) levels and decreased gamma-amino-butyric-acid (GABA) levels in the thalamus, the rostral anterior cingulate cortex (rACC) and the insula [17,18,19]. Building further on these insights, our main hypothesis is that the clinical effect of pregabalin as a medical treatment for neuropathic pain results in a decrease of cerebral glutaminergic (excitatory) neurotransmission and an increase of GABAminergic (inhibitory) neurotransmission.
The goals of this 1H-NMR study are twofold. First, we investigated the neurobiological effects of pregabalin as a treatment for neuropathic pain in four regions of interest (ROIs): the right rACC, dominant dorsolateral prefrontal cortex (DLPFC) and the bilateral thalami. Second, we evaluated the dose–response relation in these ROIs.
Methods
Eligibility and Recruitment
In this randomized, double-blind, placebo-controlled trial, we compared pregabalin with placebo for the treatment of painful diabetic polyneuropathy PDN. Twenty-seven patients are recruited in a single hospital (Universitair ziekenhuis Brussel). Patients were diagnosed with PDN based on a combination of clinical symptoms, an abnormal physical/neurological exam and an abnormal electromyography that demonstrated length-dependent distal sensory neuropathy. Patients who met the following criteria were eligible to participate in the study: diagnostic criteria for type 1 or type 2 diabetes (glycated hemoglobin [HbA1c] level ≤ 11%); stable anti-diabetic medication for 30 days prior to randomization; pain in the extremities associated with PDN for ≥ 3 months; pain intensity score of ≥ 4 on the visual analog scale (VAS). All patients were at least 18 years old and all provided informed consent prior to enrollment. Patients were excluded if: another clinically significant chronic pain condition was present (e.g. rheumatoid arthritis). Patients were also excluded from participation if they had a creatinine clearance of ≤ 60 mL/min or a contraindication for MRI (e.g. claustrophobia, incompatible pacemaker) or if they used retinotoxins. During the trial, the use of other antiepileptics, antidepressants or non-steroidal anti-inflammatory drugs was prohibited.
The study was conducted conform to the guidelines of the declaration of Helsinki (Revised Edinburgh, 2000). The protocol and the informed consent form were approved by the Ethics Committee of the Universitair Ziekenhuis Brussel. Informed consent was obtained from all individual participants included in the study. This study was registered at ClinicalTrials.gov (NCT01180608).
Randomization
All participants were randomly assigned to the placebo group or the intervention group using sealed numbered envelopes. Each patient was given his/her unique patient number, and a blinded, third party organized the study visits and distributed the medication. Pregabalin was taken orally, and all capsules had the same color, shape and taste as the placebo capsules.
Trial Oversight
Brain metabolites were measured before and after pregabalin treatment in the intervention group and compared to those in the placebo group, utilizing 1H-MRS. Following a baseline scan, patients were randomized to the placebo group or the intervention group. During the first 4 weeks of the study, the placebo group only received placebo treatment, while the intervention group received pregabalin from the start. The dosage in the intervention group escalated over a 6-week period, starting with an initial, single dose of 75 mg pregabalin daily, followed by two doses of 75 mg pregabalin per day and progressively increased until the dose was 300 mg twice daily (Fig. 1). At visit 8, the two groups were crossed-over: the pregabalin dose of the intervention group was downgraded with titration, and the placebo group was started on the same weekly titration scheme as undergone by the intervention group. In the intervention group neuroimaging sessions were held at 0 mg pregabalin (scan 1), after 1 week of 75 mg pregabalin (scan 2), after 1 week of 225 mg pregabalin (scans 3, 6) and after 1 week of 450 mg pregabalin (scans 4, 5). In the placebo group neuroimaging sessions were held at 0 mg pregabalin (scans 1, 2, 3 and 4), after 1 week of 75 mg pregabalin (scan 5) and after 1 week of 225 mg pregabalin (scan 6) (see Fig. 2).
Data Acquisition
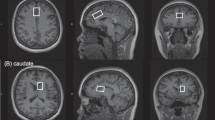
A 3T Philips MR-scanner (Achieva, software level 2.5; Philips Healthcare, Best, The Netherlands) with an eight-channel receiver head coil was used. The 1H-MR spectroscopy protocol was performed for metabolite concentration measurement at the predefined regions of interest (rACC, DLPFC, right and left thalamus) whereby regions were scanned in random order. The head of each patient was fixated with foam padding to avoid head movement during the scan. The first step was to make a three-dimensional T1-weighted image of the head as a guide for voxel placement. Spectra were acquired using a standard PRESS sequence (point-resolved spectroscopy; TE = 35 ms, TR = 2000, NS = 16). Automatic shimming was performed before each session to minimize magnetic inhomogeneity, resulting in spectra of better quality with higher signal-to-noise ratios. Two spectra were acquired for each voxel, one with water suppression and one without. The unsuppressed water signal was later used during data analysis as a reference to calculate the absolute concentrations of brain metabolites.
1H-MRS Spectroscopy
1H-MRS is a promising non-invasive technique that allows the concentration of metabolites within the human brain to be measured. It can be performed on most clinical MR-scanners without major hardware modifications. MR images map the distribution and interaction of hydrogen atoms with tissue, as opposed to 1H-MRS which analyzes the signal of hydrogen protons attached to other molecules. 1H-MRS analysis results in several peaks at different radio frequencies, whereas conventional MR images only show a single peak. Each of the 1H-MRS peaks represents a proton nucleus in a different chemical environment, or so-called spectrum. The obtained 1H-MRS data was processed using LCModel v6.3, which provided a reference spectrum that was fitted to our data. Each neurometabolite has its own unique spectral footprint from which the concentration can be calculated. Absolute concentrations were calculated using the unsuppressed water signal as a reference. Metabolites measured in this study and included in statistical analysis were: N-acetyl aspartate (NAA), Glutamate (Glu), glutamine (Gln), GABA, glucose (Glc) and myo-inositol (mINS).
Statistical Analysis
Analysis of the 1H-MR spectroscopy data on metabolite concentration was carried out using SPSS version 24 (IBM Corp., Armonk, NY, USA). A mixed design, repeated measures analysis of variance was used (ANOVA) using time as the within-subjects effect and group (placebo vs. pregabalin) as the between-subjects effect. Different analyses were performed for each brain region separately, and a post hoc analysis was conducted when a significant difference was found. Secondly a one-way between-groups ANOVA was conducted to explore the impact of pregabalin dosage on neurometabolite concentration, as measured by 1H-MRS. For this one-way ANOVA, participants were divided into four groups according to their received dose [0 mg (n = 54), 75 mg (n = 21), 225 mg (n = 31) and 450 mg (n = 20)]. Missing data due to missed visits were completed by using multiple imputations by chained equations in R.
Results
Patients
A total of 27 patients with PDN were enrolled in the study. After randomization, 13 patients were allocated to the pregabalin group and 14 patients to the placebo group. Six patients (3 from the placebo group and 3 from the pregabalin group) were excluded from the analysis because 1H-MRS was only performed at visit 1 (n = 4) or at visit 1 and 2 (n = 2). Twenty-one subjects underwent all 1H-MRS scans (10 males and 11 females). The average age of the patients in the pregabalin group and placebo group was 67 (range 42–81) years and 55 (range 43–73) years, respectively.
N-Acetylaspartate and N-Acetylaspartylglutamate
Mixed design analysis detected no statistically significant difference in combined N-acetylaspartate and N-acetylaspartylglutamate concentration over time between the pregabalin and placebo groups in any of the four regions, with the p values for rACC, the DLPFC, the left thalamus and the right thalamus being 0.817, 0.257, 0.425 and 0.654, respectively (Table 1).
A comparison of the different doses revealed that there were no statistically significant differences across the brain ROIs: rACC (p = 0.139), DLPFC (p = 0.675), left thalamus (p = 0.071) and right thalamus (p = 0.213) (Table 2).
Glutamate and glutamine
Mixed analysis showed no relevant difference in the change in the combined concentrations of Glu and Gln over time between the placebo and pregabalin group. The p values for the rACC, DLPFC, left thalamus and right thalamus were 0.448, 0.310, 0.961 and 0.404, respectively (Table 3).
A comparison of the different doses revealed that there were no statistically significant differences in any of the four ROIs (p values: rACC 0.052, left thalamus 0.093, right thalamus 0.844, DLPFC 0.077) (Table 4).
Myo-inositol
No statistically significant difference was found in the mINS concentration between the placebo and pregabalin groups over time in the brain ROIs: rACC (p = 0.410), DLPFC (p = 0.996), left thalamus (p = 0.937) and right thalamus (p = 0.844) (Table 5).
Comparison of the different doses of pregabalin revealed a significant difference in mINS concentration in the rACC (p = 0.034) and DLPFC (p = 0.049). Tukey post hoc analysis showed that the baseline group and the 450 mg group differed significantly from each other (p = 0.022) in the rACC. In the DLPFC region, a statistically significant difference was seen between the group who received 75 mg pregabalin and the group who received 450 mg (p = 0.042). Clinically, the concentration of both metabolites decreased with higher concentrations of pregabalin, with the highest decrease observed at the dose of 450 mg pregabalin (Table 6).
No statistically significant difference was found in the other ROIs, namely the left thalamus (p = 0.910) and right thalamus (p = 0.561).
Gamma-amino-butyric-acid
No statistically significant difference was found in the GABA concentration over time between the different ROIs: rACC (p = 0.157), DLPFC (p = 0.684), left thalamus (p = 0.385) and right thalamus (p = 0.509) (Table 7).
Comparison of the different doses of pregabalin revealed a statistically significant difference in the concentration of GABA in the rACC (p = 0.013). No statistically significant difference in GABA concentration was found in the other ROIs: DLPFC (p = 0.600), right thalamus (p = 0.121) and left thalamus (p = 0.578) (Table 8).
Tukey post hoc analysis revealed that there was a significant difference (p = 0.014) between the group who received a 75 mg dose of pregabalin and those who received a 225 mg dose.
Glucose
No statistically significant difference between the groups in the mean Glc concentration over time was found in the rACC (p = 0.490), DLPFC (p = 0.897) and right thalamus (p = 0.806). A statistically significant difference was found in the left thalamus (p = 0.049) (Table 9).
Comparison of the different doses of pregabalin revealed that there was no statistically significant difference in mean Glc concentration in the rACC (p = 0.180), right thalamus (p = 0.511) and DLPFC (p = 0.098). A statistically significant difference in mean Glc concentration was found in the left thalamus (p = 0.027); Tukey post hoc tests, however, revealed no statistically significant differences between the groups (Table 10).
Discussion
In this randomized controlled trial (RCT) we examined neurochemical changes in several brain neurometabolites following pregabalin administration in a population of patients with PDN. Pregabalin, an anticonvulsant, is considered to be the first-line treatment in the management of PDN, despite there being a lack of clear supporting evidence [20]. Studies on pain relief based on a dose-dependent response have shown that, in general, pregabalin administered in doses of 150 mg is ineffective while doses of 300 mg up to 600 mg result in a meaningful decrease of pain in patients suffering from PDN [20,21,22,23]. Notwithstanding increasing prescription rates, the literature on the efficacy and dose dependency of pregabalin is still conflicting [24]. Therefore, we designed this study to investigate both the efficacy of pregabalin and the effect of different doses in an objective manner. The results of many previous studies, mainly based on subjective questionnaires and pain scales, contradict each other, while only a handful of studies have examined the effect of pregabalin versus placebo [25, 26]. These two shortcomings are addressed in our study.
It is important to first clarify the efficacy and impact of the different doses of pregabalin. Tolle et al. [25] examined the effects of pregabalin in 394 patients with PDN and found a significant decrease in pain between placebo and pregabalin administered at a dose of 600 mg per day but no differences for doses of 150 mg and 300 mg per day, respectively. A pain reduction of 50% was noted in almost half of the patients treated with pregabalin, while the same reduction was also seen in one-third of placebo patients [25]. Contradictory results have also been reported, with Rosenstock et al. [26] reporting a significant superiority of 300 mg/day pregabalin versus placebo. The findings of these two studies underline the challenge of treating neuropathic pain with pregabalin, as not one dose seems to be effective for all patients. To make the matter yet more complicated, a recent RCT showed no significant pain reduction with pregabalin treatment for leg pain in patients with sciatica [27]. These results are in line with our findings, as we found no differences in metabolite concentrations between the placebo and pregabalin (intervention) groups, but did observe some differences, although small, between the different doses.
This brings us to our second point of emphasis, as all of the previous high-level RCTs describe a decrease in numerical rating scale or VAS as a successful treatment, relying on self-reporting to assess treatment effectiveness. However, some patients suffering from PDN feel no pain, while others with the exact same pathology suffer from severe pain, implying that cortical processing and individual variability in antinociceptive mechanisms play an important role in the development of pain and pain perception. Therefore, we suggest that brain imaging is an objective diagnostic tool to assess brain chemistry and treatment efficacy, providing a solution by objectifying the neural correlates of pain perception and pain modulation. Imaging modalities have already shown promise for further unraveling of the central mechanisms contributing to pain chronification [17, 28,29,30,31], which is of utmost importance as recent publications stress the underlying central mechanisms of PDN, which are not yet fully understood and seem to play an important role. [32, 33].
Our study failed to identify significant, consistent changes in the concentrations of brain metabolites between the placebo and intervention group. Several explanations for this may be considered. One possibility is that 1H-MRS is not an adequate tool to investigate cerebral changes in neurobiology. However, 1H-MRS has been proven to be a valid, non-invasive and ionizing radiation-free analytical technique, not only in chronic pain populations, but also in patients with other pathologies, such as depression and Parkinson disease [34, 35]. Furthermore, it is a widely accepted technique to assess the outlines of brain tumors when planning surgery; this approach also allows metabolites to be identified and quantified, thereby providing the necessary anatomical, physiological and chemical information [36, 37]. Various studies have thus confirmed the reliability, reproducibility and stability of 1H-MRS [37,38,39,40]. Thus, the validity of 1H-MRS should not be questioned, as it has proven to be a valuable tool to evaluate disease states or treatment protocols [41]. The second and probably the most realistic explanation for our findings may be found in our hypothesis which states that the analgesic effects of pregabalin are caused by reducing hyperexcitation in ascending pain pathways, thereby reducing dysregulation in areas of the brain associated with pain perception and restoring the inhibitory nociceptive pathways [14, 16]. Several chronic pain studies utilizing 1H-MRS have shown increased Glu levels and decreased GABA levels in the thalamus and ACC [17,18,19, 42, 43], confirming this disturbed excitation–inhibition balance and supporting our hypothesis that the clinical effect of pregabalin as a medical treatment for neuropathic pain would result in a decrease of cerebral glutaminergic (excitatory) neurotransmission and an increase in GABAminergic (inhibitory) neurotransmission. Unfortunately, the results of our study could not be used to confirm our hypothesis, suggesting that pregabalin does not restore the function of the inhibitory nociceptive pathways. In our study, we found no significant differences between patients with PDN and the controls in terms of GABA level, despite the role of GABA receptors in the modulation of pain perception. This lack of significant findings may be due to low concentrations of GABA in the brain; however, spectral editing sequences like MEGA-PRESS resolve this spectral overlap [44]. Also, NAA concentration in the thalamus has been proposed as a potential tool for investigating the efficacy of various therapies [45]. In some studies, a decrease was seen in several patient populations with neuropathic pain, with reduced NAA levels found in the DLPFC (low back pain) and the thalamus (postherpetic neuralgia, failed back surgery syndrome, complex regional pain syndrome) [17, 29]. These results underline the importance of reduced NAA levels as a chemical marker for chronic pain [29]. However, this neurometabolite did not show significant alterations over time or with dose. The same can be said for mINS and Glc, all of which have been shown to have some relationship with chronic pain but not to undergo significant alterations in concentration due to pregabalin treatment [17, 30].
The results of this study suggest that there is no objective, predictive value of 1H-MRS to identify pain reduction by pregabalin, with equal concentrations of metabolites in the different brain regions in the placebo and pregabalin groups. However, comparison of the different doses of pregabalin did reveal that higher doses induced reductions in the concentrations of mINS in the rACC and DLPFC as well as decreased concentrations of GABA in the rACC. These findings can be explained by published findings which show that higher concentrations of MI and GABA are associated with higher pain intensity ratings [46, 47].
Limitations
This study has a number limitations that may hamper generalization of the reported results. First, the sample size of our study is small, and the neuroimaging results were not correlated with clinical outcomes, such as pain intensity, quality of life, sleep or disability. Further, until now 1H-MRS has not frequently been used to assess treatment efficacy, possibly leading to measurement errors. It is also possible that 1H-MRS is not an appropriate objective tool to measure treatment efficacy based on changes in neurometabolite concentrations, as previously discussed. Lastly, when comparing the different doses, we did not compare pregabalin treatment at 600 mg per day; this could be a focus for future studies.
Conclusion
This placebo-controlled trial is the first of its kind to investigate the effect of pregabalin on brain metabolite concentration in a population suffering from PDN. Although 1H-MRS analysis showed that pregabalin does not cause significant changes in the metabolic profile of the brain, some interesting conclusion can be drawn. Although no differences were found in metabolite concentrations between the placebo and intervention group, some differences were found regarding the different doses.
References
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53.
Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–22.
Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev. 2008;24[Suppl 1]:S52–7.
Nawroth PP, Bendszus M, Pham M, et al. The quest for more research on painful diabetic neuropathy. Neuroscience. 2017. doi: https://doi.org/10.1016/j.neuroscience.2017.09.023
Vaeggemose M, Pham M, Ringgaard S, et al. Magnetic resonance neurography visualizes abnormalities in sciatic and tibial nerves in patients with type 1 diabetes and neuropathy. Diabetes. 2017;66(7):1779–88.
Vaeggemose M, Pham M, Ringgaard S, et al. Diffusion tensor imaging MR neurography for the detection of polyneuropathy in type 1 diabetes. J Magn Reson Imaging. 2017;45(4):1125–34.
Vaeggemose M, Vaeth S, Pham M, et al. Magnetic resonance neurography and diffusion tensor imaging of the peripheral nerves in patients with Charcot-Marie-Tooth Type 1A. Muscle Nerve. 2017; 56(6):E78–E84
Davis KD, Flor H, Greely HT, et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol. 2017;13(10):624–38.
Musen G, Jacobson AM, Bolo NR, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61(9):2375–9.
Climie RE, Moran C, Callisaya M, et al. Abdominal obesity and brain atrophy in type 2 diabetes mellitus. PLoS One. 2015;10(11):e0142589.
Zhang Y, Lu S, Liu C, et al. Altered brain activation and functional connectivity in working memory related networks in patients with type 2 diabetes: An ICA-based analysis. Sci Rep. 2016;6:23767.
Wu GY, Zhang Q, Wu JL, et al. Changes in cerebral metabolites in type 2 diabetes mellitus: A meta-analysis of proton magnetic resonance spectroscopy. J Clin Neurosci. 2017;45:9–13.
Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, Wang GJ. Neuroimaging the effectiveness of substance use disorder treatments. J Neuroimmune Pharmacol. 2016;11(3):408–33.
Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–64.
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73.
Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A. The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities? Trends Pharmacol Sci. 2013;34(6):332–9.
Gussew A, Rzanny R, Gullmar D, Scholle HC, Reichenbach JR. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage. 2011;54(2):1315–23.
Henderson LA, Peck CC, Petersen ET, et al. Chronic pain: lost inhibition? J Neurosci. 2013;33(17):7574–82.
Alshelh Z, Di Pietro F, Youssef AM, et al. Chronic neuropathic pain: it’s about the rhythm. J Neurosci. 2016;36(3):1008–18.
Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: an update. Am Fam Physician. 2016;94(3):227–34.
Spallone V, Lacerenza M, Rossi A, Sicuteri R, Marchettini P. Painful diabetic polyneuropathy: approach to diagnosis and management. Clin J Pain. 2012;28(8):726–43.
Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–65.
Ziegler D, Fonseca V. From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Compl. 2015;29(1):146–56.
Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411–4.
Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12(2):203–13.
Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–38.
Mathieson S, Maher CG, McLachlan AJ, et al. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376(12):1111–20.
Moens M, Marien P, Brouns R, et al. Spinal cord stimulation modulates cerebral neurobiology: a proton magnetic resonance spectroscopy study. Neuroradiology. 2013;55(8):1039–47.
Grachev ID, Thomas PS, Ramachandran TS. Decreased levels of N-acetylaspartate in dorsolateral prefrontal cortex in a case of intractable severe sympathetically mediated chronic pain (complex regional pain syndrome, type I). Brain Cogn. 2002;49(1):102–13.
Grachev ID, Fredrickson BE, Apkarian AV. Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J Neural Transm (Vienna). 2002;109(10):1309–34.
Zhao X, Xu M, Jorgenson K, Kong J. Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: a systematic review. Neuroimage Clin. 2017;13:33–8.
Tesfaye S, Selvarajah D, Gandhi R, et al. Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain. 2016;157[Suppl 1]:S72–80.
Greig M, Tesfaye S, Selvarajah D, Wilkinson ID. Insights into the pathogenesis and treatment of painful diabetic neuropathy. Handb Clin Neurol. 2014;126:559–78.
Ciurleo R, Bonanno L, Di Lorenzo G, Bramanti P, Marino S. Metabolic changes in de novo Parkinson’s disease after dopaminergic therapy: a proton magnetic resonance spectroscopy study. Neurosci Lett. 2015;599:55–60.
Shirayama Y, Takahashi M, Osone F, Hara A, Okubo T. Myo-inositol, glutamate, and glutamine in the prefrontal cortex, hippocampus, and amygdala in major depression, biological psychiatry. Cogn Neurosci Neuroimaging. 2017;2(2):196–204.
Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin N Am. 2013;23(3):359–80.
Mazuel L, Chassain C, Jean B, et al. Proton MR spectroscopy for diagnosis and evaluation of treatment efficacy in parkinson disease. Radiology. 2016;278(2):505–13.
Sharma NK, McCarson K, Van Dillen L, Lentz A, Khan T, Cirstea CM. Primary somatosensory cortex in chronic low back pain—a H-MRS study. J Pain Res. 2011;4:143–50.
Sharma NK, Brooks WM, Popescu AE, et al. Neurochemical analysis of primary motor cortex in chronic low back pain. Brain Sci. 2012;2(3):319–31.
Mao N, Fang J, Xie H, et al. Correlation between neurochemical metabolism and memory function in adolescent patients with depression: a multi-voxel (1)H magnetic resonance spectroscopy study. Psychiatry Clin Neurosci. 2016;70(4):167–74.
McGuire SA, Wijtenburg SA, Sherman PM, et al. Reproducibility of quantitative structural and physiological MRI measurements. Brain Behav. 2017;7(9):e00759.
Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci Lett. 2012;520(2):192–6.
Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA + and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. Neuroimage. 2015;105:67–75.
Mullins PG, McGonigle DJ, O’Gorman RL, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52.
Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24(1):75–9.
Pattany PM, Yezierski RP, Widerstrom-Noga EG, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR. 2002;23(6):901–5.
Kupers R, Danielsen ER, Kehlet H, Christensen R, Thomsen C. Painful tonic heat stimulation induces GABA accumulation in the prefrontal cortex in man. Pain. 2009;142(1–2):89–93.
Acknowledgements
The authors would like to thank all participants of the study.
Funding
Maarten Moens received the Lyrica Independent Investigator Research Award (LIIRA) 2010 (Pfizer), with which this study was funded. The article processing charges were funded by the author. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Compliance with Ethics Guidelines
The study was conducted conform to the guidelines of the declaration of Helsinki (Revised Edinburgh, 2000). The protocol and the informed consent were approved by the Ethics Committee of UZ Brussel. Informed consent was obtained from all individual participants included in the study.
Disclosures
Maarten Moens has received consultancy or speaking honoraria from Pfizer Inc., Medtronic and Nevro. Lisa Goudman, Mats De Jaeger, Raf Brouns, Peter Van Schuerbeek, Bart Keymeulen, Johan De Mey have nothing to disclose.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6462890.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
De Jaeger, M., Goudman, L., Van Schuerbeek, P. et al. Cerebral Biochemical Effect of Pregabalin in Patients with Painful Diabetic Neuropathy: A Randomized Controlled Trial. Diabetes Ther 9, 1591–1604 (2018). https://doi.org/10.1007/s13300-018-0460-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0460-y