Abstract
Hypoglycemia in individuals with diabetes can increase the risk of morbidity and all-cause mortality in this patient group, particularly in the context of cardiovascular impairment, and can significantly decrease the quality of life. Hypoglycemia can present one of the most difficult aspects of diabetes management from both a patient and healthcare provider perspective. Strategies used to reduce the risk of hypoglycemia include individualizing glucose targets, selecting the appropriate medication, modifying diet and lifestyle and applying diabetes technology. Using a patient-centered care approach, the provider should work in partnership with the patient and family to prevent hypoglycemia through evidence-based management of the disease and appropriate education.
Similar content being viewed by others
Introduction
Hypoglycemia is both a clinical and physiologic condition that is associated with increased morbidity and all-cause mortality in individuals with both type 1 (T1DM) and type 2 diabetes (T2DM) [1]. An increasing body of evidence suggests that hypoglycemia is harmful to patients with diabetes both immediately and over time, particularly in terms of cardiovascular health [2, 3]. While hyperglycemia can cause long-term complications, hypoglycemia can be imminently life threatening and significantly decrease the quality of life. Additionally, it is often difficult for patients to achieve the recommended glucose targets due to the fear of hypoglycemia or actual hypoglycemia. For these reasons, hypoglycemia can be one of the most difficult aspects of diabetes management from both the patient’s and healthcare provider’s perspective. However, using a patient-centered care approach and evidence-based practice, the provider can work in partnership with the patient to reduce the risk of hypoglycemia.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Epidemiology
Several large-scale clinical trials have demonstrated the incidence and prevalence of hypoglycemia in patients with both T1DM and T2DM. The prevalence of hypoglycemia in patients with T1DM has been reported to range from 62 [4] to 320 [5] episodes per 100 patient-years. Individuals with T1DM may experience an average of two symptomatic hypoglycemia episodes per week and one to three disabling, potentially life-threatening episodes per year [1, 6]. The risk of hypoglycemia in individuals with T2DM is much lower and is often associated with advanced diabetes with endogenous insulin deficiency. The prevalence of hypoglycemia in T2DM ranges from 0 [7] to 73 [5] episodes per 100 patient-years. Rates of severe hypoglycemia are more common among older adults and those with chronic conditions, such as chronic kidney disease, cardiovascular disease (CVD), congestive heart failure, depression and higher glycated hemoglobin (HbA1c) levels, as well as among those who are on insulin or take secretagogues [6].
Impact
Hypoglycemia poses an economic burden to healthcare resources. Recently published studies suggest that the average cost to the healthcare provider of treating a patient—across settings and countries—is approximately US$1200 per episode, with costs increasing by up to eightfold for the treatment of severe hypoglycemia episodes that require the patient to be admitted to hospital [8, 9].
Published data also suggest that hypoglycemia directly impairs health-related quality of life in patients with T2DM and that this impairment becomes increasingly pronounced with increasing severity and frequency of the hypoglycemic episodes [10, 11]. This relationship has not been consistently shown in adult patients with T1DM [11], but it has been more clearly documented in pediatric and adolescent patients [12].
Hypoglycemia is known to contribute to morbidity and mortality in the clinical setting of diabetes [2]. This risk is primarily cardiovascular related and is seen most often in patients with diabetes who are treated with insulin [13]. Hypoglycemia causes increases in blood pressure, stroke volume, cardiac output and myocardial contractility, all of which may cause reduced cardiovascular functioning over time [14]. The results of a 2017 study of 464 patients suggested that the crude incidence rates of CVD and death were higher in persons with hypoglycemia than in those without, even after adjusting for potential confounding variables [CVD: 10.6 vs. 5.08 per 100 person-years (PY), p < 0.001; death: 14.3 vs. 4.5 per 100 PY, p < 0.001] [3]. Several large-scale studies have confirmed the strength of this relationship, such as the ACCORD trial results (action to control CVD in diabetes) which suggested that 1% of deaths in the trial were likely caused by diabetes while 9% had hypoglycemia as a contributing factor [15]. Other studies have suggested that severe hypoglycemia may contribute to the deaths of people with T1DM in up to 10% of cases [16].
Pathophysiology
In individuals with diabetes, hypoglycemia often results from excess insulin or the inability to raise the blood glucose (BG) level through endogenous or exogenous methods [17]. In order to understand the pathophysiology of hypoglycemia, it is imperative to understand normal glucose homeostasis. The regulation of glucose is dependent on multiple systems, including the renal, hepatic, pancreatic and neuroendocrine systems. Any deficiencies within these systems (for example end-stage renal disease or liver failure) can affect the physiologic response to hypoglycemia. Multiple hormonal interactions are also involved, as shown in Table 1.
When the BG level approaches lower physiologic levels (80–85 mg/dl), a sequence of physiologic events occurs in an attempt to maintain or restore normal glucose concentration [17]. The first counter-regulatory measure is the halting of insulin production. As BG falls to 65–70 mg/dl, the alpha cells of the pancreas begin to release glucagon, an endogenous hormone that raises BG levels. At this glucose level, the body also begins to release endogenous hormones, such as epinephrine, cortisol and growth hormone, in an attempt increase BG. Finally, as the BG level drops to < 55 mg/dl, the body produces endogenous glucose from the liver to facilitate glucose recovery [1]. This chain of events is dependent on the proper functioning of the pancreatic alpha cells, liver and kidneys. The liver is responsible for 80% of gluconeogenesis while the kidneys are responsible for 20% [18]. Recurrent and frequent hypoglycemia over time can lead to hypoglycemic-associated autonomic failure (HAAF), a pathophysiologic process in which sympathoadrenal processes no longer trigger symptoms of hypoglycemia, causing potentially dangerous asymptomatic hypoglycemia [17].
Clinical Presentation and Diagnosis
In 2012, The American Diabetes Association (ADA) and the Endocrine Society assembled a workgroup to address the knowledge gaps related to the definition, implications and understanding of hypoglycemia [19]. This workgroup established parameters to define hypoglycemia and issued the statement that for individuals with diabetes “all episodes of an abnormally low plasma glucose concentration expose the individual to potential harm.” [2, 19]. Due to its highly individualized presentation, the presence of hypoglycemia may be established using Whipple’s Triad which includes: (1) signs/symptoms consistent with hypoglycemia, (2) a low plasma glucose level (typically < 70 mg/dl) and (3) the resolution of signs/symptoms of hypoglycemia followed an increase in plasma glucose level [1]. The workshop also determined five classifications of hypoglycemia, stating that these are important to recognize and document (Table 2) as they may guide management and patient education. Patients may present with a variety of signs or symptoms, and it is imperative that patients, together with family and friends, are educated on the spectrum of signs and symptoms that can indicate hypoglycemia (Table 3). It is important to recognize that hypoglycemia unawareness may be present 40–60% of the time, even in patients who report having hypoglycemia awareness [20, 21]. Patients with hypoglycemia unawareness may experience non-specific symptoms, such as waking up in the morning with a headache, a high blood glucose level in the morning (Somogyi effect) or nighttime sweating. Further, patients may exhibit vague symptoms (shown in Table 3) but may not associate them with the presence of hypoglycemia.
HAAF is a serious condition in which repeated hypoglycemic episodes fail to trigger the protective autonomic system response, leading to asymptomatic hypoglycemia. The HAAF phenomenon includes the failure of insulin levels to decrease in the presence of hypoglycemia, failure of glucagon secretion, and lack of epinephrine secretion [22]. HAAF is exacerbated by frequent or recent hypoglycemia as well as sleep or exercise [22]. When the regulatory system is working correctly, these systems ensure an adequate glucose supply to the brain in times of hypoglycemia. When HAAF is present, severe hypoglycemia may occur.
Management
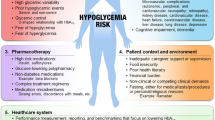
The treatment of hypoglycemia is aimed at identifying treatable and/or modifiable causes, followed by strategies for prevention and risk reduction. Important factors to consider in prevention include patient awareness of hypoglycemia, individualized glucose targets, self-monitoring of blood glucose (SMBG), diet, exercise and medication regimen.
In patients with hypoglycemic unawareness, strict avoidance of hypoglycemia by adjusting glucose goals to higher targets on a short-term basis (2–4 weeks) can allow the symptoms of hypoglycemia to return. Many patients need reassurance for this type of approach, as some patients are fearful of high glucose levels, even over the short term, and associated diabetes complications. In the patient-centered approach to glycemic control, the ADA recommends less strict glycemic goals when the benefits of tight glycemic control outweigh the risks for hypoglycemia. The recommendation is a goal of < 8.0% for patients at high risk for hypoglycemia, compared to the typical goal of < 7.0% [23]. A higher HbA1c goal is reasonable for those with hypoglycemic unawareness and chronic kidney disease, the elderly and those with CVD.
There are many risk factors and precipitants of hypoglycemia (Table 4). Renal function should be monitored, as worsening renal function is associated with a decline in insulin requirements. An estimated glomerular filtration rate (eGFR) of 10–50 ml/min should prompt a 25% reduction in insulin dosage, with a dosage reduction of 50% when the eGFR drops to < 10 ml/min [24].
Beta (β) blockers are commonly used in people with diabetes. The authors of a recent study noted that the incidence of severe hypoglycemia (confirmed BG level < 50 mg/dl) was significantly higher in those on β-blockers than those not on β-blockers [25, 26]. Moreover, β-blockers are associated with an increased risk of hypoglycemia unawareness due to the β-blockade that blunts symptoms of hypoglycemia in addition to lowering blood pressure [27]. For patients at high risk of hypoglycemia, it may be prudent to change anti-hypertensive agents.
A visit to a certified diabetes educator to review glucose logs along with diet and activity logs can help discern important glucose trends and hypoglycemia triggers. For those patients who are working on losing weight, a 24-h diet recall should be included to assess whether insulin mealtime doses should be altered. Weight loss improves insulin sensitivity and may lead to a necessary reduction in insulin doses.
A new exercise routine or a change in type or intensity of activity will increase insulin sensitivity, glucose utilization and the “lag effect” during which muscle glucose stores are replenished after exercise. This creates a glucose utilization/insulin dose mismatch and can increase the risk for hypoglycemia. Lowering the insulin dose or increasing food intake for the meal before the planned exercise are strategies to prevent hypoglycemia, and both interventions may be necessary [28]. For patients engaged in lifestyle modifications, such as increased physical activity and dietary changes, it may be necessary to reduce the insulin dose by 10–20%. Guidelines for carbohydrate intake related to exercise for people with diabetes are shown in Table 5.
Many of the new agents to treat diabetes are less likely to cause hypoglycemia than the older classes of medications. In addition to metformin, glucagon-like peptide-1 (GLP-1) agonists, dipeptidyl peptidase-4 (DPP-IV) inhibitors and sodium–glucose cotransporter 2 (SGLT-2) inhibitors are all excellent choices for people who are at risk of hypoglycemia. Older medication classes, such as sulfonylureas and meglitinides, should generally be avoided by patients who are at high risk for hypoglycemia.
An understanding of the pharmacokinetic profiles of the various insulin preparations is important when the aim is to modify insulin dosing to prevent hypoglycemia. In addition, there are insulins that are associated with less frequent hypoglycemia, and thus a change in insulin preparation may help reduce the frequency of low glucose readings. The pharmacokinetics of common insulin types is shown in Table 6 [29]. Most notably, the intermediate-action insulin isophane, also known as NPH, and regular insulin are associated with more frequent hypoglycemic episodes than the long-acting glargine, detemir and degludec insulins, particularly at night. The authors of a 2017 study comparing degludec insulin (a once-daily ultra-long-acting insulin) to glargine insulin in over 7000 patients at high risk for CVD reported that compared to patients using glargine, patients on degludec showed a 40% reduction in severe hypoglycemia and a 53% reduction in nocturnal hypoglycemia (p < 0.001) [23].
Continuous glucose monitors (CGMs) have revolutionized the treatment and prevention of hypoglycemia. These monitors measure interstitial glucose levels every 5 min, thereby providing real time data for patient use. CGMs are not meant to replace SMBG, and most models need to be calibrated twice daily with SMBG to assure accuracy. CGMs also identify important glucose trends, such impending hypoglycemia, that allow for early treatment and prevention of hypoglycemia. CGM data can be downloaded and reviewed online or in a clinic setting to help providers identify trends to allow for more accurate medication modification. The CGMs also alarm at night, alerting patients and families to hypoglycemia. There is a strong body of evidence noting that the use of CGM results in less frequent hypoglycemic episodes when compared to conventional SMBG, while improving and stabilizing overall glycemic control [30].
Patients find that CGM can help improve their quality of life and self-efficacy in managing hypoglycemia. However, CGMs are less accurate during times of rapid glucose excursions (such as right after a meal) [30]. Patients may find the false alarms and need for calibration to be annoying (alarm fatigue), and the cost of a CGM may be prohibitive for some patients [31]. Patients likely to benefit from CGMs include individuals with required manual dexterity to insert and operate the sensor system and individuals with multiple risk factors for hypoglycemia (older age, chronic kidney disease, autonomic neuropathy, CVD). Starting in 2017, Medicare in the USA will cover CGMs for selected patients [32].
Insulin pumps (continuous subcutaneous insulin infusion) have long been recognized as a tool that can decrease hypoglycemia while improving glycemic control. CGMs are also integrated into insulin pump technology and include an alarm and automatic 2-h suspension of the insulin infusion for hypoglycemia, which is particularly helpful at night. The sensor-augmented pumps can reduce the frequency of hypoglycemic episodes while maintaining good glucose control [33]. Results from the initial closed-loop trial (the Pivatol trial) of 124 patients with T1DM suggested that the MiniMed 670G/Enlite 3 system (Medtronic, Dublin, Ireland) kept the participants within the target range 72% of the time (compared to 67% for those not using the system) and was associated with a 44% reduction in time spent with low BG (< 70 mg/dl) and a 40% decline in severe low BG (< 50 mg/dl) [34]. Other strategies for preventing hypoglycemia include a CGM-augmented pump that infuses both insulin and glucagon [35]. This type of integrated system would be one step closer to creating the much desired “artificial pancreas.”
Conclusion
Hypoglycemia causes harm to people with diabetes, creating cardiovascular impairment and an increased risk of cardiovascular morbidity and all-cause mortality [19]. Further, hypoglycemia significantly impacts the quality of life of patients with diabetes and can limit optimal glucose control. A patient-centered approach is imperative to achieve optimal glucose control while avoiding hypoglycemia and its harmful effects. A patient-centered approach is one that is based on shared medical decision-making among the patient, family and healthcare provider and uses individualized approaches to problem solving and diabetes management planning. Education aimed at recognizing the signs and symptoms of hypoglycemia is imperative for both patients and families. Appropriate teaching includes individual risk factors, prevention, and treatment of hypoglycemia. In addition, healthcare providers must work diligently with patients and families to identify and eradicate hypoglycemia by using appropriate glucose targets and medications and modifying lifestyle.
References
Cryer P. Hypoglycemia in diabetes: pathophysiology, prevalence, and prevention. American Diabetes Association:Alexandria, VA. 2012.
International Hypoglycaemia Study Group. Minimizing hypoglycemia in diabetes. Diabetes Care. 2015;38(8):1583–91.
Lee AK, Warren B, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. Association of severe hypoglycemia with cardiovascular disease and all-cause mortality in older adults with diabetes: the atherosclerosis risk in communities (ARIC) study. Circulation. 2017;135:A43.
DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991;90(4):450–9.
UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–7.
Pathak RD, Schroeder EB, Seaquist ER, Zeng C, Lafata JE, Thomas A, Karter AJ. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in US integrated health care delivery systems: 2005–2011. Diabetes Care. 2016;39(3):363–70.
Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, VA CSDM Group. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM): results of the feasibility trial. Diabetes Care. 1995;18(8):1113–23.
Alexiu C, Kirkland SW, Jelinski S, Chuck A, Campbell S, Rowe BH. P112: cost of hypoglycemia associated with diabetes mellitus: a systematic review of the literature. CJEM. 2016;18(S1):S115.
Foos V, Varol N, Curtis BH, Boye KS, Grant D, Palmer JL, McEwan P. Economic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United States. J Med Econ. 2015;18(6):420–32.
Marrett E, Radican L, Davies MJ, Zhang Q. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes. 2011;4(1):251.
McCoy R, Van Houten H, Ziegenfuss J, Shah N, Wermers R, Smith S. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19(5):792–9.
McGill DE, Levitsky LL. Management of hypoglycemia in children and adolescents with type 1 diabetes mellitus. Curr Diabetes Rep. 2016;16(9):88.
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38(2):316–22.
Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34[Suppl 2]:S132–7.
Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Longterm mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298–305.
Kreider KE, Padilla BI, Pereira K. Hypoglycemia in diabetes: challenges and opportunities in care. J Nurse Pract. 2017;13(3):228–34.
Cano N. Bench-to-bedside review: glucose production from the kidney. Crit Care. 2002;6(4):317.
Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
Reno CM, Litvin M, Clark AL, Fisher SJ. Defective counterregulation and hypoglycemia unawareness in diabetes: mechanisms and emerging treatments. Endocrinol Metab Clin North Am. 2013;42(1):15.
Bakatselos SO. Hypoglycemia unawareness. Diabetes Res Clin Pract. 2011;93:S92–6.
Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369(4):362–72.
Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, Moses A. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017; 377:723–32.
Snyder RW, Berns JS. Reviews: use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004; 17(5):365–70.
Tsujimoto T, Sugiyama T, Shapiro MF, Noda M, Kajio H. Risk of cardiovascular events in patients with diabetes mellitus on B-blockers. Hypertension. 2017;70:103–10.
Murad MH, Coto-Yglesias F, Wang AT, et al. Drug-induced hypoglycemia: a systematic review. J Clin Endocrinol Metab. 2009;94(3):741–5.
Zaharieva DP, Riddell MC. Prevention of exercise-associated dysglycemia: a case study-based approach. Diabetes Spectr. 2015;28(1):55–62.
Donner T. Insulin—pharmacology, therapeutic regimens and principles of intensive insulin therapy. 2015. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. South Dartmouth (MA): MDText.com, Inc.; 2000.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18[Suppl 2]:S2-3–13.
Centers for Medicare and Medicaid Services. Classification of therapeutic continuous glucose monitors as “Durable Medical Equipment” under Medicare Part B. https://www.cms.gov/Regulations-and-Guidance/Guidance/Rulings/Downloads/CMS1682R.pdf (2017). Accessed 23 Oct 2017.
Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–32. doi:10.1056/NEJMoa1303576.
Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, Bergenstal RM. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–63.
Ranjan A, Schmidt S, Madsbad S, Holst JJ, Nørgaard K. Effects of subcutaneous, low-dose glucagon on insulin-induced mild hypoglycaemia in patients with insulin pump treated type 1 diabetes. Diabetes Obes Metab. 2016;18(4):410–8.
Boyle PJ, Zrebiec J. Management of diabetes-related hypoglycemia. South Med J. 2007;100(2):183–94.
Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Dequist ER, Service FJ. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709–28.
Horton ES, Shahar J. Exercise for fitness. In: Beaser RS (ed) Joslin’s diabetes deskbook: a guide for primary care providers. 3rd ed. Boston: Joslin Diabetes Center; 2014. p. 155–85.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Kathryn Evans Kreider, Katherine Pereira, Blanca I. Padilla have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/A6CCF060352E1427.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Evans Kreider, K., Pereira, K. & Padilla, B.I. Practical Approaches to Diagnosing, Treating and Preventing Hypoglycemia in Diabetes. Diabetes Ther 8, 1427–1435 (2017). https://doi.org/10.1007/s13300-017-0325-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-017-0325-9