Abstract
Introduction
Regular self-monitoring of blood glucose (SMBG) is recommended as an integral part of therapy for all patients with diabetes treated with insulin. In the current study, the effects on glycemic control of taking 7-point SMBG profiles and using a diabetes management system (DMA) on a smartphone were investigated.
Methods
In a 12-week, open-label, multicenter, observational study, 51 patients [26 with type 1 diabetes mellitus (T1DM) and 25 with type 2 diabetes mellitus (T2DM)] were instructed to perform SMBG at least seven times a day using DMA combined with the iBGStar® SMBG system. HbA1c was measured at regular visits to the study sites. Patients reviewed and managed their data as well as their treatment on their own and there were no further assistance or treatment recommendations. Adverse events (AEs) were recorded throughout.
Results
Overall, mean (SD) change from baseline in HbA1c at week 12 was −0.46 (0.57)% [−5 (6) mmol/mol (p < 0.0001)]. The change in HbA1c was observed in patients with T1DM [−0.27 (0.45)% (−3 [5] mmol/mol; p = 0.0063)] and T2DM [−0.65 (0.62)% (−7 [7] mmol/mol; p < 0.0001)]. The change in HbA1c was not correlated with an increased number of hypoglycemic events (blood glucose less than 55 mg/dL). The majority of AEs were symptomatic hypoglycemic events (42 events; nine patients).
Conclusions
Glycemic control can be improved, without receiving any recommendations or advice on insulin dose, by performing daily 7-point SMBG profiles and using electronic documentation with a smartphone app. These results must be confirmed in a larger controlled trial, but they already strengthen the importance of structured SMBG in diabetes therapy.
Funding
Sanofi.
Similar content being viewed by others
Introduction
Patients with type 1 diabetes (T1DM) require insulin therapy, as do many with type 2 diabetes (T2DM), to control their blood glucose concentration. An integral part of their therapy is regular self-monitoring of blood glucose (SMBG) in order to adjust insulin dose(s) to achieve target blood glucose concentration(s). The American Diabetes Association (ADA) guidelines encourage individuals to perform SMBG at each meal and bedtime [1]. Various approaches have been employed to try to enhance patients’ motivation and adherence to their regimen and thereby improve glycemic control. These extend from the use of a paper-based tool to record blood glucose data [2, 3] to digital decision support tools [4], connectivity [5], and other features for “smart” SMBG devices [6,7,8].
The iBGStar® Blood Glucose Meter is a diagnostic device for quantitative SMBG measurements. The iBGStar® Diabetes Manager Application (DMA) is a digital logbook and diabetes management tool for iPhone and iPod Touch. The DMA allows for collection of information such as 7-point SMBG profiles, physical exercise, general physical conditions, meals, glucose-lowering drugs, and insulin doses. It can be used alone or with an iBGStar® device connected to an iPod or iPhone where BG measurements from the meter are automatically transferred to the DMA. In the current pilot study, the iBGStar® DMA system was used solely to record daily 7-point SMBG values without providing any recommendations/advice about insulin doses by a healthcare professional. The 12-week study investigated the effects of performing daily 7-point SMBG profiles on HbA1c in patients with T1DM or insulin-treated T2DM.
Methods
Patients
Patients, age at least 18 years, with T1DM or insulin-treated T2DM who were taking basal insulin alone or in combination with preprandial insulin were eligible. They had to be willing and able to perform 7-point SMBG using iBGStar® and to use the DMA on an iPod Touch on a daily basis. They had to provide signed written informed consent. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Study Design
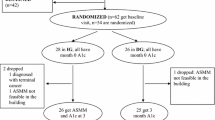
This was a 12-week, open-label, uncontrolled, multicenter, observational study conducted in Germany (Fig. 1). At the first visit (week 0), patients gave their written informed consent, and data on their demography, body weight, height, and diabetes history were recorded in an electronic case report form (e-CRF). They were provided with the iBGStar® SMBG system and an iPod Touch 4 with preinstalled DMA version 2.2 and given detailed instructions on their use and how to perform SMBG. Additional training was provided at each subsequent visit if deemed necessary.
Patients continued with their background orally administered or injectable antihyperglycemic drug(s). Patients were instructed to measure BG at least seven times a day (before and after breakfast, lunch, and dinner, at bedtime, and before any snack) using the iBGStar® SMBG system combined with the DMA. Beside SMBG data, patients were instructed to manually collect information in the DMA on their daily diabetes routines and to record any complaints about the DMA. Data from the DMA could be downloaded to the e-CRF by sending an email to the data management group; this allowed data to be collected other than during the semi-monthly onsite visits (weeks 2–12) and to check for compliance.
Blood samples were collected at each study site visit and analyzed for HbA1c at a local laboratory. Adverse events (AEs) and serious AEs, including hypoglycemia, were recorded retrospectively throughout the study. Hypoglycemia was defined as an event during which typical symptoms of hypoglycemia (such as sweating, blurred vision, trembling, or lack of concentration) were accompanied by measured plasma glucose less than 55 mg/dL (less than 3.1 mmol/L). Importantly, patients reviewed and managed their data as well as their treatment on their own; no further assistance or treatment recommendations were provided by the investigators or the DMA.
Statistical Analysis
Descriptive statistics were provided for demographic, diabetes history, safety, and laboratory data. Change in HbA1c from start to week 12 was analyzed by t test. Linear regression was used to analyze the relationship of the change in HbA1c to the number of episodes of SMBG less than 55 mg/dL (less than 3.1 mmol/L).
Results
Patient Disposition and Characteristics
Fifty-one patients (T1DM, n = 26; T2DM, n = 25) enrolled in the study and 50 completed the study; one patient with T1DM discontinued because of a serious AE (tibia fracture). The mean age of patients with T2DM was greater than those with T1DM (62.0 vs 45.6 years), as was their body mass index (33.5 vs 25.0 kg/m2), while the duration of diabetes was shorter (15.7 vs 21.9 years) (Table 1). All patients with T1DM were taking a basal plus preprandial insulin, while 13 patients (52.0%) with T2DM were taking a basal plus preprandial insulin, 11 (44.0%) were taking only a basal insulin, and one (4.0%) was taking only a preprandial insulin. The mean dose of basal insulin was greater in patients with T2DM than in those with T1DM (36.6 vs 26.8 U), as was the dose of preprandial insulin (52.3 vs 30.7 U). The most common other glucose-lowering drug for patients with T2DM was metformin [19 patients (73.1%)].
Glycemic Control
The mean (SD) number of daily SMBG measurements during the study was 7.1 (1.5), with no significant differences observed between patients with T1DM vs T2DM or between those taking basal vs basal plus preprandial insulin. For all patients (N = 50), mean (SD) HbA1c declined from 7.5 (1.0)% [58 (11) mmol/mol] at the start of the study to 7.1 (0.9)% [54 (10) mmol/mol] at 12 weeks (Fig. 2a). The change from baseline to week 12 [−0.46 (0.57)% (−5 [6] mmol/mol)] was significant (p < 0.0001).
HbA1c at start and 12 weeks and change in HbA1c at 12 weeks. a All patients (n = 50). b Patients with T1DM (n = 25) vs T2DM (n = 25). c Patients with T2DM taking basal insulin alone (n = 13) or patients with T1DM or T2DM taking basal + prandial insulin (n = 36). T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
The reduction in HbA1c was observed in both patients with T1DM (n = 25) and T2DM (n = 25). The mean (SD) starting HbA1c for patients with T1DM was 7.6 (1.0)% [60 (11) mmol/mol] and 7.4 (1.1)% [57 (12) mmol/mol] for those with T2DM (Fig. 2b). The change in HbA1c from the start to week 12 was −0.27 (0.45)% [−3 (5) mmol/mol] in patients with T1DM (p = 0.0063) and −0.65 (0.62)% [−7 (7) mmol/mol] in those with T2DM (p < 0.0001). The reduction in HbA1c occurred in patients who were taking basal insulin alone (n = 13) as well as those taking basal plus preprandial insulin (n = 36) (Fig. 2c). The change at week 12 was −0.80 (0.78)% [−9 (9) mmol/mol] in patients taking basal insulin (p = 0.0029) and −0.35 (0.44)% [−4 (5) mmol/mol] in those taking basal plus preprandial insulin (p < 0.0001).
A total of 33 patients had an HbA1c above 7% at study entry. Of these, 16 patients (48.5%) reached an HbA1c level below 7% at the end of the study.
Hypoglycemia
Eight patients with T1DM (32.0%) reported 41 hypoglycemic events and one patient with T2DM (4.0%) reported a single event. Of the 42 events, 15 (35.7%), 21 (50.0%), and 6 (14.3%) were of mild, moderate, and severe intensity, respectively. One event was a serious AE as it required hospitalization in a patient with low HbA1c, diabetes duration of 44 years, and regular consumption of alcohol. All counted hypoglycemic events were accompanied by symptoms such as sweating, blurred vision, trembling, or lack of concentration. There was no correlation between hypoglycemic events and change in HbA1c (Fig. 3). The slope of the regression line was not significantly different from zero (r 2 = 0.0473; p = 0.1294).
Safety
A total of 24 patients (47.1%) experienced at least one AE during the study. The intensity of AEs was classified as mild for 13 patients (25.5%), moderate for 11 (21.6%), and severe for 7 (13.7%). The most common AEs were nasopharyngitis [7 patients (13.7%)] and hypoglycemia [9 (17.6%)]. Of the 78 AEs reported, 72 (92.3%) were resolved at the end of the study. Six patients (11.8%) experienced at least one serious AE. The serious AEs were cardiac arrest, posterior capsule rupture in the right eye, cholecystitis, tibia fracture, hypoglycemia, bladder cancer, and diabetic foot. All of the AEs were assessed by the investigator as having no relation to the medical procedure.
Discussion
In this observational study, glycemic control was improved, without any further assistance from healthcare providers, by performing daily 7-point SMBG profiles and using electronic therapy documentation provided by the iPod/DMA. Subgroup analyses showed the reduction occurred in patients with either T1DM or T2DM, those with T2DM taking basal insulin alone, or those with T1DM or T2DM taking basal plus prandial insulin, indicating that the reduction was not confined to only one type of diabetes or insulin regimen. However, there is the caveat of not having a control group for any of the groups. The reduction in HbA1c was not correlated with an increase in hypoglycemic episodes.
While the number of times the patients performed SMBG before starting the study was not recorded, it may be assumed that it was less than the seven times a day during the study. The increase in the number of daily SMBG measurements and additionally having the results recorded in the DMA may have led to greater attention by the patients to their therapy and subsequently to improved glycemic control, even in the absence of any assistance with insulin dose adjustments from healthcare professionals. The frequency of SMBG measurements has been shown to be associated with improvement in HbA1c in patients with T1DM [9], as well as in insulin-treated patients with T2DM [10].
When patients with non-insulin-treated T2DM were trained to use a paper tool to collect and interpret 7-point SMBG profiles, a significant reduction of 0.3% HbA1c was observed compared with the active control group [2]. Similarly, patients with non-insulin-treated T2DM who underwent intensive structured monitoring with 4-point glycemic profiles had a significantly greater 0.12% HbA1c reduction than the active control group [3]. In recent years, the number of devices connected to smartphone applications has increased [11,12,13]. However, only a limited number of studies have been reported evaluating the potential of SMBG devices connected to electronic data management systems on smartphones [8, 14].
A limitation to our study is that the observation period was only 3 months. We do not have follow-up data and it remains difficult to estimate long-term compliance with this type of self-monitoring. Longitudinal data on SMBG usage is scarce. A reduction of 1.1 strips in daily monitoring frequency was shown during a 3-year follow-up study in insulin-treated patients with a very low strip utilization (1.94/day) at study entry, but nothing is known about the type of insulin treatment, patient training on SMBG usage, or proportion of type 1 and 2 patients [15]. After 2 years only 20% of patients with T2DM continued to measure their blood glucose daily as required during a lifestyle intervention over the first 12 weeks including diurnal SMBG profiles and event-driven measurements [16]. In both studies, patients only had SMBG devices but no diabetes app with analysis features. Thus we speculate that compliance may be higher in such a setting also during the long term.
The small sample size of our study is also a limitation and the results must be confirmed in a larger controlled trial. However, the current results do strengthen the importance of SMBG in diabetes therapy.
References
American Diabetes Association. Glycemic targets. Diabetes Care. 2016;39:S39–46. doi:10.2337/dc16-S008.
Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262–7.
Bosi E, Scavini M, Ceriello A, et al. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care. 2013;36:2887–94.
Bajaj HS, Venn K, Ye C, Aronson R. Randomized trial of long-acting insulin glargine titration Web Tool (LTHome) vs enhanced usual therapy of glargine titration (INNOVATE trial). Diabetes Technol Ther. 2016;18:610–5.
Livongo® Health. Livongo website. https://www.livongo.com/. Accessed 3 April 2017.
diaTribe Foundation. Sanofi’s iBGStar: iPhone/iPad Touch + Blood Glucose Meter = A better testing experience. diaTribe website. https://diatribe.org/sanofis-ibgstar-iphoneipod-touch-blood-glucose-meter-better-testing-experience. Accessed 3 April 2017.
Rao A, Hou P, Golnik T, Flaherty J, Vu S. Evolution of data management tools for managing self-monitoring of blood glucose results: a survey of iPhone applications. J Diabetes Sci Technol. 2010;4:949–57.
Clements MA, Staggs VS. A mobile app for synchronizing glucometer data. Impact on adherence and glycemic control among youths with type 1 diabetes in routine care. J Diabetes Sci Technol. 2017. doi:10.1177/1932296817691302.
Minder AE, Albrecht D, Schafer J, Zulewski H. Frequency of blood glucose testing in well educated patients with diabetes mellitus type 1: how often is enough? Diabetes Res Clin Pract. 2013;101:57–61.
Schutt M, Kern W, Krause U, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114:384–8.
Bailey TS, Wallace JF, Pardo S, et al. Accuracy and user performance evaluation of a new, wireless-enabled blood glucose monitoring system that links to a smart mobile device. J Diabetes Sci Technol. 2017. doi:10.1177/1932296816680829.
Christiansen M, Greene C, Pardo S, et al. A new, wireless-enabled blood glucose monitoring system that links to a smart mobile device. Accuracy and user performance evaluation. J Diabetes Sci Technol. 2017. doi:10.1177/1932296817691301.
Harvey C, Koubek R, Begat V, Jacob S. Usability evaluation of a blood glucose monitoring system with a spill-resistant vial, easier strip handling, and connectivity to a mobile app: improvement of patient convenience and satisfaction. J Diabetes Sci Technol. 2016;10:1136–41.
Shah V, Hiatt W, Gottlieb P, Beatson C, Snell-Bergeon J, Garg S. Role of mobile technology to improve diabetes care in adults with type 1 diabetes: the REMOTE-TID study. Diabetes Technol Ther. 2015;17(S1):A24–5.
Karter AJ, Parker MM, Moffet HH, et al. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care. 2006;29:1757–63.
Kempf K, Kruse J, Martin S. ROSSO-in-praxi follow-up: long-term effects of self-monitoring of blood glucose on weight, hemoglobin A1c, and quality of life in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2012;14:59–64.
Acknowledgements
The study and article processing charges were funded by Sanofi. Editorial support was provided by Tom Claus, PhD, of PAREXEL and funded by Sanofi. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Johan Sieberan is an employee of Sanofi-Aventis Deutschland GmbH. Frank Flackeis is an employee of Sanofi-Aventis Deutschland GmbH. Manuela Link and Cornelia Haug have nothing to disclose. Guido Freckmann has received speakers’ honoraria or consulting fees from Abbott, Ascensia, Bayer, Berlin-Chemie, Becton–Dickinson, Dexcom, LifeScan, Menarini Diagnostics, Novo Nordisk, Roche, Sanofi, Sensile, and Ypsomed.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D90CF0606DD678BC.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sieber, J., Flacke, F., Link, M. et al. Improved Glycemic Control in a Patient Group Performing 7-Point Profile Self-Monitoring of Blood Glucose and Intensive Data Documentation: An Open-Label, Multicenter, Observational Study. Diabetes Ther 8, 1079–1085 (2017). https://doi.org/10.1007/s13300-017-0306-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-017-0306-z