Abstract
Introduction
The glucagon-like peptide-1 (GLP-1) receptor agonist class has grown in the last decade, with several agents available in the UK. However there is currently a paucity of evidence regarding the relative cost-effectiveness of liraglutide 1.2 mg versus other daily administered GLP-1 receptor agonists, due to a lack of head-to-head trial data. Therefore the present analysis was performed, using results from a network meta-analysis (NMA), to compare the cost-effectiveness of three currently available daily administered GLP-1 receptor agonists for treatment of diabetes in the UK setting.
Methods
A validated and published diabetes model was used to make long-term projections of clinical outcomes and direct costs (2015 GBP) for patients receiving liraglutide 1.2 mg once-daily, exenatide 10 μg twice daily and lixisenatide 20 μg once-daily. Treatment effects were taken from an NMA evaluating the efficacy of GLP-1 receptor agonists and were applied in a cohort based on the Liraglutide Effect and Action in Diabetes 6 (LEAD-6) trial. Costs and utilities were based on published sources.
Results
Liraglutide 1.2 mg was associated with improved quality-adjusted life expectancy versus exenatide [9.19 versus 9.17 quality-adjusted life years (QALYs)] and lixisenatide (9.19 versus 9.12 QALYs). Improvements were driven by benefits in glycemic control, leading to a reduced incidence of diabetes-related complications. Liraglutide 1.2 mg was associated with reduced costs versus exenatide (GBP 36,394 versus GBP 36,547) and lixisenatide (GBP 36,394 versus GBP 36,496), with cost savings as a result of complications avoided entirely offsetting increased acquisition costs. Based on the projected outcomes, liraglutide was found to be dominant over both exenatide and lixisenatide.
Conclusion
Liraglutide 1.2 mg is likely to be considered cost-effective versus alternative daily administered GLP-1 receptor agonists for treatment of type 2 diabetes in the UK.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is a complex and progressive disease that is associated with a significant clinical and economic burden globally. Currently 415 million people have diabetes worldwide, with type 2 diabetes representing approximately 90% of cases, and it is estimated that this will rise to 642 million in 2040 [1]. In the United Kingdom (UK) the prevalence of diabetes is 6.2%, with 3.3 million diagnosed patients [2]. In 2013, over 50,000 hospital admissions and approximately 5000 deaths were attributable to the disease. Across the UK, one in seven hospital beds is occupied by a patient with diabetes, and in some hospitals this can be as high as 30% of beds [3]. People with diabetes are at twice the risk of experiencing a cardiovascular event during their lifetime compared with the general population, and diabetes is the most common cause of renal failure (the primary cause in over 25% of patients) [4, 5]. The risk of micro- and macrovascular complications can be reduced through maintaining glycemic control and managing other risk factors such as body weight, hypertension and hyperlipidemia [6,7,8].
Diabetes is also associated with a significant economic burden in the UK, with type 2 diabetes comprising the majority of this. In 2011 the annual direct cost of type 2 diabetes was estimated to be GBP 8.8 billion, with indirect costs of GBP 13 billion. It is projected that direct and indirect costs will increase to GBP 15.1 billion and GBP 20.5 billion, respectively, by 2035 [9]. It has been estimated that drug costs make up less than 10% of the total cost burden of diabetes in the UK and that the predominant driver of the economic burden is diabetes-related complications [9].
The glucagon-like peptide-1 (GLP-1) receptor agonist class represents an attractive treatment option for patients with type 2 diabetes and their use has grown in the last decade. Within the UK, three alternative daily administered GLP-1 receptor agonists are available: exenatide 10 μg twice daily (BID, approved by the European Medicines Agency in 2006), liraglutide 1.2 or 1.8 mg once daily (approved in 2009) and lixisenatide 20 μg once daily (approved in 2013). The GLP-1 receptor agonists act through activation of the GLP-1 receptor, which is present within a number of tissues, resulting in a range of physiological effects. These include stimulation of glucose-dependent insulin secretion, inhibition of glucagon release, delaying gastric emptying, and increasing satiety [10, 11]. This broad mechanism of action gives the GLP-1 receptor agonists a number of favorable treatment characteristics. As well as improved glycemic control, GLP-1 receptor agonists are associated with weight loss, reductions in blood pressure, and low risk of hypoglycemia [10, 11]. This has been shown to result in long-term benefits, with data from the randomized, double-blinded Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial (NCT01179048) showing that liraglutide 1.8 mg was associated with statistically significant reductions in the risk of cardiovascular disease, death from any cause, and nephropathy compared with placebo over a median of 3.8 years of follow-up, and a retrospective analysis of 39,275 patients has also suggested that exenatide may be associated with a reduced risk of cardiovascular events than other treatment options [12, 13].
While numerous studies have been conducted that have compared GLP-1 receptor agonists with other therapy options such as sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, and insulin glargine, only three published studies have directly compared daily administered GLP-1 receptor agonists for a period greater than 24 weeks [14,15,16,17,18,19,20,21,22]. The Liraglutide Effect and Action in Diabetes 6 (LEAD-6) trial (NCT00518882) compared liraglutide 1.8 mg with exenatide 10 μg BID in patients with type 2 diabetes failing to achieve glycemic targets on metformin and/or sulfonylurea [20]. Liraglutide 1.8 mg reduced mean glycated hemoglobin (HbA1c) significantly more than exenatide 10 μg BID (estimated treatment difference −0.33; 95% confidence interval −0.47 to −0.18; p < 0.0001), and more patients receiving liraglutide achieved an HbA1c target of <7% (54% versus 43%, respectively). Both liraglutide 1.8 mg and exenatide 10 μg BID were associated with similar weight loss (−3.24 and −2.87 kg, respectively). Rates of hypoglycemia were low in both treatment arms, but liraglutide 1.8 mg was associated with a statistically significant reduction in minor hypoglycemic events (1.93 versus 2.60 events per patient per year; p = 0.0131).
Liraglutide 1.8 mg and lixisenatide 20 μg were compared in patients with type 2 diabetes not meeting glycemic targets on metformin monotherapy in the LIRA-LIXI trial (NCT01973231) [22]. Liraglutide 1.8 mg was associated with a greater reduction in HbA1c (estimated treatment difference −0.62%; 95% confidence interval −0.80% to −0.44%; p < 0.0001) and greater proportions of patients achieving HbA1c targets of <7% and ≤6.5%. Both interventions were associated with similar reductions in blood pressure and body weight, and had similar safety profiles.
The GetGoal-X study (NCT00707031) compared lixisenatide 20 μg with exenatide 10 μg BID in patients with type 2 diabetes failing to achieve glycemic control on metformin monotherapy [21]. The two therapies were found to be comparable, with lixisenatide 20 μg found to be non-inferior to exenatide 10 μg BID in terms of reduction in HbA1c (estimated treatment difference 0.17%; 95% confidence interval 0.033 to 0.297%) and similar proportions of patients achieved an HbA1c target of <7% (48.5% with lixisenatide and 49.8% with exenatide). Weight loss was also similar with lixisenatide 20 μg and exenatide 10 μg BID (−2.96 kg with lixisenatide and −3.98 kg with exenatide). No patients in either treatment arm experienced a severe hypoglycemic event, but a lower proportion of patients experienced non-severe events with lixisenatide 20 μg (2.5%) than with exenatide 10 μg (7.9%).
Whilst there are clinical trials comparing liraglutide 1.8 mg with other daily administered GLP-1 receptor agonists, to date, there is a paucity of head-to-head trial evidence for liraglutide 1.2 mg versus other daily administered GLP-1 receptor agonists. Recently, a network meta-analysis comparing the efficacy and safety of daily administered GLP-1 receptor agonists for the treatment of insulin naïve patients with type 2 diabetes has been published [23]. This network meta-analysis based on 13 randomized, controlled trials represents the first assessment of the relative efficacy of currently available daily administered GLP-1 receptor agonists with liraglutide 1.2 mg included.
The healthcare budget within the UK is coming under increased pressure, with an ageing population and limited increases in healthcare spending planned over the coming years. The aim of health economic evaluation is to ensure efficient allocation of resources such that the National Health Service (NHS) achieves the maximum healthcare gains across the population within the restrictions of the limited budget. A number of health economic evaluations comparing GLP-1 receptor agonists with other classes of therapy have been published [24,25,26,27]. However, to date, only one long-term modeling analysis assessing the cost-effectiveness of alternative daily administered GLP-1 receptor agonists in the UK setting has been conducted. This analysis assessed the cost-effectiveness of liraglutide 1.8 mg and exenatide 10 μg BID in the UK setting based on the LEAD-6 study, and concluded that liraglutide 1.8 mg was cost-effective [28]. The cost-effectiveness of liraglutide 1.2 mg once daily has not been assessed versus either exenatide 10 μg BID or lixisenatide 20 μg once-daily, reflecting the lack of head-to-head trial evidence for liraglutide 1.2 mg versus other GLP-1 receptor agonists.
The aim of the present analysis was to explore this area further and to compare the long-term cost-effectiveness of currently available daily administered GLP-1 receptor agonists for which there is no head-to-head trial evidence. The study evaluated the cost-effectiveness of liraglutide 1.2 mg, exenatide 10 μg BID and lixisenatide 20 μg, for treatment of patients with type 2 diabetes in the UK setting based on the results of a network meta-analysis [23].
Methods
Model Description
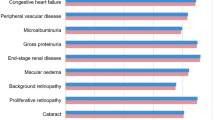
The analysis was performed using the IMS CORE Diabetes Model (IMS Health, Basel, Switzerland), the architecture, assumptions, features and capabilities of which have been previously published [29]. The model is a validated, non-product specific diabetes policy analysis tool and is based on a series of inter-dependent sub-models that simulate the complications of diabetes (angina, myocardial infarction, congestive heart failure, stroke, peripheral vascular disease, diabetic retinopathy, macular edema, cataract, hypoglycemia, ketoacidosis, lactic acidosis, nephropathy and end-stage renal disease, neuropathy, foot ulcer and amputation, and non-specific mortality). Each sub-model has a semi-Markov structure and uses time, state, time-in-state and diabetes type-dependent probabilities derived from published sources (full details available from Palmer et al. 2004 [29]). Monte Carlo simulation using tracker variables overcomes the memory-less properties of the standard Markov model, and allows interconnectivity and interaction between individual complication sub-models. Long-term outcomes projected by the model have been validated against real life data in 2004 and more recently in 2014 [30, 31].
A simulated cohort of 1000 patients was run through the model 1000 times for each simulation (base case and sensitivity analyses). Mean values and standard deviations were generated for long-term outcomes (life expectancy, quality-adjusted life expectancy, cumulative incidence of diabetes-related complications, time to onset of diabetes-related complications, direct medical costs). The time horizon was set to patient lifetimes in the base case (50 years) to capture all relevant long-term complications and associated costs, thereby assessing their impact on life expectancy and quality-adjusted life expectancy. Future costs and clinical benefits were discounted symmetrically by 3.5% per annum in line with published health economic guidance for the UK [32].
Simulated Cohort
The baseline cohort of the analysis was based on the LEAD-6 trial, a 26-week, open-label, parallel-group study comparing liraglutide 1.8 mg once daily with exenatide 10 μg BID [20]. Participants were eligible to take part if they had type 2 diabetes with HbA1c of 7–11%, were aged 18–80 years, with body mass index (BMI) of 45.0 kg/m2 or less, and were on stable treatment with metformin and/or sulfonylurea. Exclusion criteria were previous treatment with insulin, previous treatment with exenatide or liraglutide, impaired liver or renal function, clinically significant cardiovascular disease, retinopathy or maculopathy requiring acute treatment, uncontrolled hypertension (≥180/100 mmHg), or cancer. The mean age of the cohort was 56.7 years (standard deviation 10.3 years), with a mean HbA1c of 8.2% (standard deviation 1.0%) and a mean duration of diabetes of 8.2 years (standard deviation 6.0 years). This cohort was chosen as it is likely to represent patients that are receiving GLP-1 receptor agonists in clinical practice in the UK. The IMS CORE Diabetes generates the cohort to be applied in the modeling analysis based on the LEAD-6 data by sampling around the mean values based on standard deviations for each parameter.
Estimation of Treatment Effects
The study aimed to assess the cost-effectiveness of daily administered GLP-1 receptor agonists where there is currently no head-to-head trial evidence (liraglutide 1.2 mg, exenatide 10 μg BID and lixisenatide 20 μg). Clinical data were taken, therefore, from a network meta-analysis of 13 randomized, controlled trials evaluating the efficacy and safety of daily administered GLP-1 receptor agonists for the treatment of insulin naïve patients with type 2 diabetes [23]. Change from baseline in HbA1c, BMI and systolic blood pressure were taken from the network meta-analysis. For each of the therapies, the network meta-analysis provided the mean differences in each parameter, versus each of the other therapies. Therefore to calculate the treatment effects to be applied in the modeling analysis, these differences were applied to a reference treatment. Treatment differences versus liraglutide 1.8 mg identified in the network meta-analysis (Table 1) were applied to the liraglutide 1.8 mg arm of the LEAD-6 study to give the treatment effects applied in the first year of the analysis in each of the treatment arms (Table 2). No data were available to inform the systolic blood pressure change in the lixisenatide 20 μg arm and therefore this was matched to the liraglutide 1.2 mg arm. This is likely to be a conservative approach as liraglutide 1.2 mg was associated with a greater reduction in systolic blood pressure than exenatide 10 μg BID, and therefore in the absence of data for lixisenatide 20 μg the most favourable change was assumed. Moreover, it is likely that the alternative GLP-1 receptor agonists are associated with comparable reductions in systolic blood pressure [33, 34]. Liraglutide 1.2 mg was associated with a statistically significant reduction in HbA1c compared to exenatide 10 μg BID and lixisenatide 20 μg, but differences in systolic blood pressure and BMI were not statistically significant.
Treatment Intensification and Long-term Parameter Progression
Patients were assumed to receive GLP-1 receptor agonist therapy for 3 years before intensifying to receive neutral protamine Hagedorn (NPH) insulin for the remainder of the simulation. This assumption recognizes that intensification to basal insulin will be required to maintain glycemic control, and is in line with previously published cost-effectiveness analyses and submissions to the National Institute for Health and Care Excellence (NICE) [24, 35]. Alternative assumptions around treatment switching were tested in sensitivity analyses (see below). Following application of the treatment effects in the first year of the analysis, HbA1c and systolic blood pressure were assumed to follow the natural progression algorithms built into the IMS CORE Diabetes Model, based on the United Kingdom Prospective Diabetes Study (UKPDS) [29]. BMI was assumed to remain constant whilst patients received GLP-1 receptor agonist therapy, and returned to baseline on intensification to NPH insulin.
Costs and Utilities
Costs were accounted from an NHS healthcare payer perspective in the UK in 2015 pounds sterling (GBP). Costs of therapy captured the respective GLP-1 receptor agonists, concomitant metformin, needles and self-monitoring of blood glucose testing (three tests per week in all treatment arms). Costs of treating diabetes-related complications (Table 3) were identified through literature review, with costs inflated to 2015 values using the Hospital and Community Health Services price index were necessary [36]. Health-related quality of life utilities associated with diabetes-related complications were derived from previous cost-effectiveness evaluations of GLP-1 receptor agonists carried out in the UK setting (Table 4) [24, 25].
Sensitivity Analyses
A number of sensitivity analyses were conducted to identify key drivers of outcomes in the base case analysis. The influence of time horizon on the model outcomes was assessed by running the analyses over 20 years. It should be noted that this analysis does not capture all long-term outcomes, as not all patients were dead at the end of the simulation. The effect of discount rates on future costs and clinical outcomes was investigated through analyses in which they were set (symmetrically) to 0% and 6% per annum, in line with guidance for the UK setting [32]. The key drivers of clinical outcomes were assessed by abolishing the differences in individual clinical parameters between the liraglutide 1.2 mg arm and the comparator arms. Additional analyses with only the statistically significant differences between liraglutide 1.2 mg and each of the two comparators applied were conducted. The influence of treatment switching was assessed in analyses with treatment switching pushed back to 5 years in all treatment arms, and triggered by patients exceeding an HbA1c threshold of 8.0% (resulting in treatment switching in different years for the alternative GLP-1 receptor agonists). Analyses were also conducted where patients switched to insulin glargine, rather than NPH insulin, after 3 and 5 years of GLP-1 receptor agonist therapy. An alternative set of cost of complications data were applied, based on the NICE liraglutide submission, with all values inflated to 2015 GBP [32, 36]. In February 2014, an update to the IMS CORE Diabetes Model incorporating data from the UKPDS 82 was released, and an analysis using this version of the model has been run for each comparison [37]. Whilst a validation study of the revised model has been published, the model proprietors suggest that the update is used in a sensitivity analysis, with the previous version being used in the base case. An analysis was conducted with clinical and cost outcomes projected for liraglutide 1.34 mg, as this reflects the mean dose of liraglutide received in the UK, based on a review of prescription data [38].
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Base Case Analysis
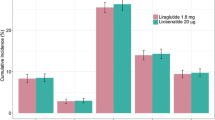
The base case analysis found that liraglutide 1.2 mg was associated with benefits in life expectancy and quality-adjusted life expectancy versus other daily administered GLP-1 receptor agonists, at 13.73 years and 9.19 quality-adjusted life years (QALYs), respectively (Table 5). Lixisenatide 20 μg was associated with the lowest life expectancy (13.67 years) and quality-adjusted life expectancy (9.12 QALYs). Differences between the three therapy arms resulted from differences in the cumulative incidence of end-stage diabetes-related complications, driven by differences in glycemic control. Liraglutide 1.2 mg was associated with the lowest cumulative incidence of microvascular and macrovascular diabetes-related complications, followed by exenatide 10 μg BID, followed by lixisenatide 20 μg (Fig. 1). The mean time to onset of complications also differed between the treatment arms. Time free of all complications with liraglutide 1.2 mg was increased by 0.1 years versus exenatide 10 μg BID (4.8 years) and 0.3 years versus lixisenatide 20 μg (4.6 years). The largest benefit with liraglutide 1.2 mg was seen in the delayed onset of neuropathy, delayed by 0.2 years compared with exenatide 10 μg BID and 0.3 years compared with lixisenatide 20 μg. Exenatide 10 μg BID was associated with the highest mean direct costs over patient lifetimes (Fig. 2) at GBP 36,547, followed by lixisenatide 20 μg (GBP 36,496) and liraglutide 1.2 mg (GBP 36,394). The analysis found that liraglutide 1.2 mg was associated with the highest treatment costs, due to the higher acquisition costs of the medication over the first three years of the analysis. However, liraglutide 1.2 mg was associated with the lowest costs of treatment of diabetes-related complications (GBP 27,337 versus GBP 27,712 with exenatide 10 μg BID and GBP 28,023 with lixisenatide 20 μg). This resulted in liraglutide 1.2 mg being associated with cost savings of GBP 153 versus exenatide 10 μg BID and GBP 103 versus lixisenatide 20 μg, as costs savings as a result of avoided diabetes-related complications entirely offset the increased treatment costs.
Liraglutide 1.2 mg was associated with improved clinical outcomes at a cost saving versus exenatide 10 μg BID and lixisenatide 20 μg. Therefore liraglutide 1.2 mg was considered dominant over both alternative daily administered GLP-1 receptor agonists.
Sensitivity Analyses
Sensitivity analyses identified that the key driver of improved clinical outcomes with liraglutide 1.2 mg was a greater reduction in HbA1c than with exenatide 10 μg BID and lixisenatide 20 μg (Table 6). Abolishing the HbA1c difference versus each of the two alternative GLP-1 receptor agonists resulted in reduced life expectancy and increased direct costs for liraglutide 1.2 mg versus exenatide 10 μg BID and lixisenatide 20 μg. Abolishing the systolic blood pressure benefit (only possible in the comparison with exenatide 10 μg BID) showed that this also drove an improvement in clinical outcomes with liraglutide 1.2 mg, but the effect was not as large as for HbA1c. Applying only the statistically significant difference of HbA1c in each comparison (with all other inputs set to the value in the comparator arm) did not change the conclusion that liraglutide 1.2 mg was dominant over exenatide 10 μg BID and lixisenatide 20 μg.
Reducing the time horizon to 20 years resulted in reduced quality-adjusted life expectancy and direct costs in all three treatment arms. While liraglutide 1.2 mg remained dominant versus exenatide 10 μg BID and lixisenatide 20 μg, increases in quality-adjusted life expectancy and cost savings were not as large as in the base case analysis. This reflects the long-term benefits of improved glycemic control during treatment with liraglutide 1.2 mg. Altering the discount rates also reflected the long-term benefits associated with liraglutide 1.2 mg. Applying a discount rate of 0% resulted in an increased clinical benefit and increased cost savings over exenatide 10 μg BID and lixisenatide 20 μg.
Clinical outcomes were stable when alternative assumptions around treatment switching were evaluated, but cost outcomes were sensitive to changes. Assuming that patients receiving insulin glargine rather than NPH insulin resulted in only minor changes in the incremental cost outcomes, and the conclusions of the analyses did not change. Assuming patients received GLP-1 receptor agonist therapy for 5 years before switching to NPH insulin resulted in increased costs in the liraglutide 1.2 mg arm compared with both exenatide 10 μg BID and lixisenatide 20 μg, with ICERs of GBP 1939 and GBP 3693 per QALY gained respectively. When patients received insulin glargine, rather than NPH insulin, after 5 years of GLP-1 receptor agonist therapy ICERs were increased to GBP 2195 and GBP 3854 per QALY gained versus exenatide 10 μg BID and lixisenatide 20 μg, respectively. Use of an HbA1c threshold of 8.0% to trigger treatment intensification resulted in treatment switching occurring in year 3 with lixisenatide 20 μg, and year 4 with liraglutide 1.2 mg and exenatide 10 μg BID. Liraglutide 1.2 mg was associated with smaller cost savings versus exenatide 10 μg BID, and increased costs of GBP 523 versus lixisenatide 20 μg. Differences in total direct costs in these scenarios were driven predominantly by changes in the cost of acquiring the GLP-1 receptor agonists due to the variation in duration of therapy.
Applying an alternative set of cost of complications data resulted in lower direct costs in all three treatment arms. The higher acquisition cost of liraglutide 1.2 mg remained completely offset by cost savings as a resulted of avoided complications in the comparison with exenatide 10 μg BID, but increased costs were only partially offset in the comparison with lixisenatide 20 μg. An ICER of GBP 2458 per QALY gained was calculated for liraglutide 1.2 mg versus lixisenatide 20 μg.
Using the updated UKPDS risk equations to calculate the risk of clinical events resulted in increased quality-adjusted life expectancy in all treatment arms, but differences between the treatment arms remained similar. Costs were reduced with all therapies. Liraglutide 1.2 mg and exenatide 10 μg BID were associated with comparable costs, and liraglutide 1.2 mg was associated with increased costs of GBP 146 versus lixisenatide 20 μg.
Analysis of liraglutide 1.34 mg, reflecting the mean dose received in clinical practice in the UK, resulted in ICERs of GBP 5120 and GBP 2513 per QALY gained versus exenatide 10 μg BID and lixisenatide 20 μg, respectively [38]. This reflected the increased clinical benefit and the increased cost of the higher dose.
Discussion
The present analysis found that treatment with liraglutide 1.2 mg once daily was associated with improved long-term clinical outcomes versus exenatide 10 μg BID and lixisenatide 20 μg once daily in the UK. The network meta-analysis on which the analysis was based identified that liraglutide 1.2 mg was associated with a greater reductions in HbA1c and systolic blood pressure than exenatide 10 μg BID and lixisenatide 20 μg [23]. These improvements drove greater reductions in the cumulative incidence of diabetes-related complications, increased the time to onset of diabetes-related complications, and resulted in increased life expectancy and quality-adjusted life expectancy in long-term projections. Liraglutide 1.2 mg was associated with higher acquisition costs, but lower costs of treating diabetes-related complications. This resulted in liraglutide 1.2 mg being associated with reduced overall direct costs versus exenatide 10 μg BID and lixisenatide 20 μg, with liraglutide 1.2 mg found to be dominant over exenatide 10 μg BID and lixisenatide 20 μg. Therefore liraglutide 1.2 mg is likely to be considered a cost-effective treatment option for patients with type 2 diabetes in the UK setting, compared with alternative daily administered GLP-1 receptor agonists.
Metformin remains the first-line pharmacological therapy for patients with type 2 diabetes in the UK. Clinicians have a number of treatment options for a second agent to be added to metformin, and a previous analysis has assessed the cost-effectiveness of liraglutide versus sulfonylureas and dipeptidyl peptidase 4 (DPP-4) inhibitors in the UK setting [24]. This analysis found that liraglutide 1.2 mg was cost-effective versus both comparators, but the key drivers of the improved clinical outcomes varied (greater reductions in systolic blood pressure and BMI versus sulfonylurea and greater reductions in HbA1c and BMI versus DPP-4 inhibitors). Given the multifactorial clinical benefits of daily administered GLP-1 receptor agonists (liraglutide, exenatide BID and lixisenatide) and their potential to address many of the clinical needs of patients with type 2 diabetes, the present analysis sought to address the question of which of these agents is most cost-effective. The present analysis is the first to evaluate the cost-effectiveness of daily administered GLP-1 receptor agonists available to clinicians in the UK, and suggests that liraglutide 1.2 mg may be the most cost-effective daily administered GLP-1 receptor agonist.
In the UK, GLP-1 receptor agonists are recommended for patients with type 2 diabetes who have a BMI of 35 kg/m2 or higher and specific psychological or other medical problems associated with obesity, or patients who have a BMI lower than 35 kg/m2 and for whom insulin therapy would have significant occupational implications or weight loss would benefit other significant obesity-related comorbidities. The present analysis aimed to explore the cost-effectiveness of liraglutide 1.2 mg versus other once daily administered GLP-1 receptor agonists where there is no head-to-head trial evidence. The analysis did not include liraglutide 1.8 mg, as there are studies available to directly to compare cost-effectiveness with exenatide 10 μg BID and lixisenatide 20 μg [20, 22]. A potential limitation of the study may be the use of a network meta-analysis to inform the clinical parameters of the modeling analysis. In the absence of head-to-head data, the network meta-analysis represents the best available source of evidence to compare the three daily administered GLP-1 receptor agonists. The use of evidence synthesis is becoming more widely accepted as part of economic evaluation of new interventions, and guidelines on best practice methods are available, with particular relevance to the UK setting [23, 39]. However, a number of the comparisons included in the network meta-analysis were represented by only one trial and this limited data pool may increase uncertainty around the outcomes. Furthermore, a number of the studies included in the network meta-analysis were open-label due to the nature of comparing an injectable agent with an oral agent, or comparing injectable agents with different injection frequencies. Additionally, the network meta-analysis did not provide all inputs required for an analysis using the IMS CORE Diabetes Model. For example, the network meta-analysis calculated the proportion of patients experiencing hypoglycemic events rather than rates of hypoglycemic events (which may affect quality of life) as required by the IMS CORE Diabetes Model, and no data were provided on changes in serum lipids (which may drive differences in cardiovascular outcomes), although these parameters are unlikely to vary notably between GLP-1 receptor agonists and inclusion of thee variables is unlikely to change the conclusions of the analysis. While the use of a network meta-analysis may be considered a weakness, the conclusions of the network meta-analysis are likely to be robust. Across the phase 3 liraglutide trial program, both the 1.2 and 1.8 mg doses have consistently been shown to be associated with reductions in HbA1c of 1% or more and significant weight loss [40]. The network meta-analysis found that liraglutide 1.2 mg was associated with a larger reduction in HbA1c than exenatide 10 μg BID and lixisenatide 20 μg, which was statistically significant in both cases.
An additional limitation may be that not all treatments a patient with type 2 diabetes not achieving glycemic control on metformin monotherapy may receive were included in the analysis. Additional therapy options include sodium-glucose co-transporter type 2 (SGLT-2) inhibitors or weekly GLP-1 receptor agonists. These therapy options were not included in the cost-effectiveness analysis, as the purpose of the network meta-analysis on which it was based was to compare the clinical efficacy of daily administered GLP-1 receptor agonists and it, therefore, did not contain data for these interventions. Additional cost-effectiveness analyses of other therapies patients not achieving glycemic control on metformin monotherapy may receive is required to further inform decision making and resource allocation.
A further limitation of the present analysis is the reliance on short-term data in making long-term predictions of outcomes over time horizons of up to 50 years. However, this is a limitation inherent to most cost-effectiveness modeling studies and, despite this, such studies represent one of the best available options for making estimates of long-term clinical and economic outcomes in the absence of long-term clinical data. As a result there is unavoidable uncertainty around how well the modeling analysis represents the real world. The present study aims to minimize this limitation, through use of a recently validated model, which has been accepted by a number of reimbursement authorities, to conduct the analysis and by conducting extensive sensitivity analyses to examine the effects of alternative inputs on long-term projections [31].
Conclusions
The present study represents the first long-term cost-effectiveness analysis of alternative daily administered GLP-1 receptor agonists in the UK setting. The analysis suggests that, based on changes in clinical parameters from a network meta-analysis, treatment with liraglutide 1.2 mg was associated with improved life expectancy and quality-adjusted life expectancy. These improved clinical outcomes came at a cost saving versus exenatide and lixisenatide. Whilst liraglutide was associated with the highest acquisition costs, this was entirely offset by cost savings as a result of avoided treatment of diabetes-related complications. Liraglutide 1.2 mg once daily was projected to be dominant over exenatide 10 μg BID and lixisenatide 20 μg once daily for insulin naïve patients with type 2 in the UK setting.
References
International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels: International Diabetes Federation; 2015.
Health and Social Care Information Centre. Quality and outcomes framework—prevalence, achievements and exceptions report. http://www.hscic.gov.uk/catalogue/PUB15751/qof-1314-report.pdf.
Health and Social Care Information Centre. National Diabetes Inpatient Audit 2012. http://www.hscic.gov.uk/diabetesinpatientaudit.
Collaboration Emerging Risk Factors. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
UK Renal Registry 16th Annual Report: 2013. https://www.renalreg.org/wp-content/uploads/2014/09/Report2013.pdf.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of Type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000:321;405–12.
Webb DR, Khunti K, Gray LJ, Srinivasan BT, Farooqi A, Wareham N, Griffin SC. Davies MJ; ADDITION-Leicester study group. Intensive multifactorial intervention improves modelled coronary heart disease risk in screen-detected Type 2 diabetes mellitus: a cluster randomized controlled trial. Diabet Med. 2012;29(4):531–40.
Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93.
Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the United Kingdom, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–62.
Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283.
Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther. 2015;6(3):239–56.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, Hussein MA. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–5.
Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD. Colagiuri S; LEAD-1 SU study group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268–78.
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M. Matthews DR; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90.
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M. Bode B; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–81.
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM. Simó R; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met + SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–55.
Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Thomsen AB, Søndergaard RE, Davies M; 1860-LIRA-DPP-4 Study Group. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447–56.
Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762–8.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47.
Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, Gerich JE. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36(10):2945–51.
Nauck M, Rizzo M, Johnson A, Bosch-Traberg H, Madsen J, Cariou B. Once-daily liraglutide versus lixisenatide as add-on to metformin in Type 2 diabetes: a 26-week randomized controlled clinical trial. Diabetes Care. 2016;39(9):1501–9.
Lorenzi M, Ploug UJ, Vega G, Jansen JP. Liraglutide vs other daily GLP-1 analogous in people with type 2 diabetes: a network meta-analysis. Abstract presented at ISPOR 18th Annual European Congress, 7–11 November 2015, Milan, Italy.
Davies MJ, Chubb BD, Smith IC, Valentine WJ. Cost-utility analysis of liraglutide compared with sulphonylurea or sitagliptin, all as add-on to metformin monotherapy in Type 2 diabetes mellitus. Diabet Med. 2012;29(3):313–20.
Beaudet A, Palmer JL, Timlin L, Wilson B, Bruhn D, Boye KS, Lloyd A. Cost-utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ. 2011;14(3):357–66.
Woehl A, Evans M, Tetlow AP, McEwan P. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled type 2 diabetes in the United Kingdom. Cardiovasc Diabetol. 2008;7:24.
Ray JA, Boye KS, Yurgin N, Valentine WJ, Roze S, McKendrick J, Tucker DM, Foos V, Palmer AJ. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin. 2007;23(3):609–22.
National Insitute for Health and Care Excellence. NICE technology appraisal guidance [TA203]: liraglutide for the treatment of type 2 diabetes mellitus: The manufacturer’s submission. https://www.nice.org.uk/guidance/ta203/chapter/the-manufacturers-submission.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, Lammert M, Spinas GA. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (Types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):5–26.
Palmer AJ, Roze S, Valentine W, Minshall ME, Foos V, Lurati FM, Lammert M, Spinas GA. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):27–40.
McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–24.
National Institute for Health and Care Excellence. The guidelines manual 2012. Available at: http://www.nice.org.uk/article/PMG6/chapter/1%20Introduction. Accessed 18 Aug 2015.
Schmidt LJ, Habacher W, Augustin T, Krahulec E, Semlitsch T. A systematic review and meta-analysis of the efficacy of lixisenatide in the treatment of patients with type 2 diabetes. Diabetes Obes Metab. 2014;16(9):769–79.
Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin-Méry A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15(7):642–9.
National Institute for Health and Care Excellence. Liraglutide for the treatment of type 2 diabetes mellitus: NICE technology appraisal guidance 203. https://www.nice.org.uk/guidance/ta203. Accessed 18 Aug 2015.
Personal Social Services Research Unit. Unit costs of health and social care 2013. Available at: http://www.pssru.ac.uk/project-pages/unit-costs/2013/. Accessed 19 Dec 2014.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33.
Divino V, DeKoven M, Hallinan S, Varol N, Wirta SB, Lee WC, Reaney M. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014;5(2):499–520.
Dias SW, N.J.; Sutton, A.J.; Ades, A.E. NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision Making, 2011; last updated April 2012. Available from: http://www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf. Accessed 28 Aug 2015.
Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract. 2012;97(1):27–42.
National institute for Health and Care Excellence. Clinical Guideline 48. MI: secondary prevention (CG48). Appendix B. Available at: http://www.nice.org.uk/CG48. Accessed 13 Mar 2014.
Dyer MT, Goldsmith KA, Khan SN, Sharples LD, Freeman C, Hardy I, Buxton MJ, Schofield PM. Clinical and cost-effectiveness analysis of an open label, single-centre, randomised trial of spinal cord stimulation (SCS) versus percutaneous myocardial laser revascularisation (PMR) in patients with refractory angina pectoris: the SPiRiT trial. Trials. 2008;9:40.
Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. CMAJ. 2009;180(4):400–7.
National Institute for Health and Care Excellence. Technology Appraisal 94: statins for the prevention of cardiovascular events, Appendix B. Available at: http://www.nice.org.uk/guidance/TA94. Accessed 31 Jul 2014.
Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. Pharmacoeconomics. 2003;21(Suppl 1):43–50.
Department of Health. NHS reference costs 2012-13. Available at: https://www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013. Accessed 13 Mar 2014.
National Institute for Health and Care Excellence. Chronic kidney disease (CG73). Appendix 2. Available at: http://www.nice.org.uk/nicemedia/live/12069/42116/42116.pdf. Accessed 13 Mar 2014.
Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med. 2003;20(6):442–50.
Meads C, Hyde C. What is the cost of blindness? Br J Ophthalmol. 2003;87(10):1201–4.
Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care. 2002;11(2):70–4.
Clarke P, Gray A, Holman R. Estimating utility values for health statenices of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Mak. 2002;22(4):340–9.
Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637.
Evans M, Khunti K, Mamdani M, Galbo-Jorgensen CB, Gundgaard J, Bogelund M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90.
Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–30.
Acknowledgements
The study and article processing charges have been supported by funding from Novo Nordisk Health Care AG. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Donna Ashley is an employee of Novo Nordisk. Qing Ye is an employee of Novo Nordisk. Barnaby Hunt is an employee of Ossian Health Economics and Communications. William Valentine is an employee of Ossian Health Economics and Communications. Ossian received consulting fees from Novo Nordisk to fund the present analysis.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C337F06011EDC215.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hunt, B., Ye, Q., Valentine, W.J. et al. Evaluating the Long-Term Cost-Effectiveness of Daily Administered GLP-1 Receptor Agonists for the Treatment of Type 2 Diabetes in the United Kingdom. Diabetes Ther 8, 129–147 (2017). https://doi.org/10.1007/s13300-016-0219-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0219-2