Abstract
Introduction
For people with type 2 diabetes (T2DM) inadequately controlled with oral antidiabetic drugs (OADs), evidence from both randomized controlled trials (RCTs) and real-world studies has demonstrated that treatment intensification with liraglutide offers effective glycemic control, weight reduction, and a lower risk of hypoglycemia compared to treatment intensification with insulin or additional OADs. Sodium glucose cotransporter 2 (SGLT-2) inhibitors are a new class of OADs that have also been shown to be effective in T2DM patients inadequately controlled with OADs. Currently there are no head-to-head RCTs comparing these to liraglutide.
Methods
We aimed to evaluate the relative efficacy, using network meta-analysis (NMA), of treatment intensification with liraglutide and SGLT-2 inhibitors people with T2DM who have been treated with metformin (alone or in combination with SU, DPP-4, and TZD). We performed a systematic literature review to identify relevant RCTs comparing liraglutide (1.2 and 1.8 mg), canagliflozin (100 and 300 mg), empagliflozin (10 and 25 mg), or dapagliflozin (5 and 10 mg) to placebo. To strengthen the indirect evidence base, we also included non-placebo RCTs where sitagliptin (100 mg) was the active comparator. Bayesian NMA was performed on the following outcomes to assess the relative efficacy and safety of interventions: reduction (change) in HbA1c, weight, and fasting plasma glucose (FPG) as well as proportion reaching target HbA1c (<7%), and risk of hypoglycemia. Doses for each intervention were considered separately.
Results
A total of 16 RCTs were identified. All trials were similar with respect to important baseline characteristics and study design. Both doses of liraglutide were generally statistically significantly superior to the SGLT-2s with respect to change from baseline in HbA1c and FPG as well as odds of reaching target HbA1c <7%. For weight, canagliflozin 300 mg was superior to liraglutide 1.2 mg, and SGLT-2s were generally associated with larger change from baseline in weight. For risk of major or minor hypoglycemia, no differences were found between treatments.
Conclusions
Compared to SGLT-2 inhibitors, liraglutide offers improvement in HbA1c and FPG. Reductions in weight are likely comparable between liraglutide and SGLT-2s. Liraglutide did not differ from SGLT-2s in terms of risk of hypoglycemia. Given the lack of head-to-head evidence, this analysis provides valuable insight into the comparative outcomes of liraglutide versus SGLT-2 inhibitors.
Similar content being viewed by others
Introduction
The worldwide burden of diabetes is increasing, with a projected prevalence of 366 million by 2030 [1]. A number of management strategies are currently in use for type 2 diabetes mellitus (T2DM), a progressive metabolic disorder characterized by a reduction in insulin production and secretion as well as increased insulin resistance. Typically, initial treatment for people with T2DM involves lifestyle changes. Subsequent stages typically involve treatment with oral antidiabetic drugs (OADs), such as metformin or sulfonylureas (SUs), or GLP-1 receptor agonists (RAs). Clinical guidelines published by the American Association of Clinical Endocrinologists and American College of Endocrinology in 2016 recommend lifestyle therapy plus GLP-1 receptor agonist monotherapy as an acceptable alternative to initial therapy with metformin for select patients with recent-onset T2DM or with mild hyperglycemia (HbA1c <7.5%) [2]. However, the 2016 American Diabetes Association guidelines maintain that metformin is the preferred initial treatment option, unless contraindicated. The addition of a second oral agent, a GLP-1 receptor agonist, or basal insulin is recommended for patients where management with maximum-dose non-insulin monotherapy is inadequate after 3 months of treatment [3].
Liraglutide is a once-daily GLP-1 RA that has been evaluated and shown to be effective and safe in randomized controlled trials (RCTs) and real-world studies throughout the T2DM treatment spectrum. Previous reviews have highlighted the improved glycemic control and favorable safety profile of the GLP-1 RA class of drugs [4,5,6,7,8].
Sodium-glucose cotransporter 2 inhibitors (SGLT-2 inhibitors) are a novel class of once-daily OADs that target the kidneys, increasing urinary glucose excretion. As these treatments act independently of insulin, they have been found to be effective throughout the T2DM spectrum [9,10,11]. Evidence from several reviews of the clinical literature suggests that SGLT-2 inhibitors improve glycemic control while also offering a favorable weight profile and a low risk of hypoglycemia [9, 11,12,13,14].
Liraglutide and SGLT-2 inhibitors have not been compared against each other in head-to-head trials. Through a network meta-analysis (NMA) framework, which permits estimation of the relative treatment effects of interventions that have not been compared against each other in an RCT, we assessed the relative efficacy of liraglutide against SGLT-2 inhibitors among people with T2DM with inadequate glycemic control despite treatment with metformin alone or in combination with other OADs.
Methods
Study Identification
A systematic literature review encompassing intervention search terms for liraglutide, canagliflozin, dapagliflozin, and empagliflozin for T2DM was conducted from inception to October 2014. For the purposes of the current analysis, the literature search was updated with terms specific to liraglutide and SGLT-2 inhibitors to identify recent relevant publications. In all iterations of the literature review, MEDLINE, Embase, and the Cochrane Register of Controlled Trials (CENTRAL) were searched to identify relevant RCTs of adults (≥18 years) with T2DM who have inadequate HbA1c control despite a stable regimen of metformin (alone or in combination with SU, TZD, or DPP-4). RCTs assessing liraglutide 1.2 mg, liraglutide 1.8 mg, canagliflozin 100 mg, canagliflozin 300 mg, dapagliflozin 5 mg, dapagliflozin 10 mg, empagliflozin 10 mg, or empagliflozin 25 mg were eligible for inclusion in the current analysis. Eligible active comparators were any agent from the TZD or DPP-4 class as well as basal insulin. The full PICOS (Population, Interventions, Comparisons, Outcomes, Study) design statement is described in Supporting Information 1. The search strategies are presented in Supporting Information 2.
Data Extraction and Outcomes
Two reviewers, independently and in duplicate, extracted data on study and patient characteristics, treatment details, and outcomes of interest from each included publication. The following baseline patient characteristics were extracted: age (years); sex (% female); race/ethnicity (% white, % black, % Asian, % Hispanic); weight (kg); BMI (kg/m2); HbA1c (%); and duration of diabetes (years). For the continuous outcomes of interest (HbA1c, weight, fasting plasma glucose (FPG)) the change from baseline (CFB) was extracted whenever available, along with corresponding sample size, and measures of uncertainty for all relevant intervention groups. For publications where CFB was not reported, the CFB was calculated as the difference in value at baseline and end of treatment.
Statistical Analyses
For each outcome of interest, we performed a Bayesian NMA to compare the efficacy and safety of liraglutide to those of SGLT-2 inhibitors. NMA methodology allows for the combination of direct and indirect evidence [15] and this approach is widely accepted by decision-makers in the context of health technology assessment [16]. For the continuous outcomes, change from baseline (CFB) in HbA1c, FPG, and weight, a regression model with a normal likelihood distribution and identity link was used, while a binomial likelihood distribution and logit link were used for the proportion of patients meeting HbA1c target as well as the proportion of patients experiencing hypoglycemia.
In order to evaluate the consistency between direct and indirect comparisons, edge-splitting was performed [17]. This iterative technique involves splitting the available evidence into direct and indirect information for each comparison for which both are available. For each treatment comparison in the network, two relative treatment effects are estimated: one with pairwise comparison models based on direct comparisons and one based on an NMA of the remaining studies using indirect evidence only. After this assessment of inconsistency, the Bucher test was performed for each three-sided loop [18]. Both fixed-effects and random-effects models were fitted to the data using Bayesian Markov Chain Monte Carlo (MCMC) methods, and these models were compared using the deviance information criterion (DIC) to determine whether the random- or fixed-effects model was more appropriate [19].
For analyses of continuous outcomes, we present mean differences (MDs) and 95% credible intervals (CrIs) from the posterior distribution of relative treatment effects. For the proportion of patients meeting HbA1c target values, results are presented as odds ratios (ORs) with corresponding 95% CrIs. In line with Bayesian statistics, statistical superiority (akin to statistical significance with frequentist statistics) was asserted when the 95% credible intervals precluded the null effect (i.e., 0.0 for MDs and 1.0 for ORs). All analyses were performed using R version 3.0.3 and OpenBUGS version 3.2.3 (OpenBUGS Project Management Group).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Study Identification and Selection
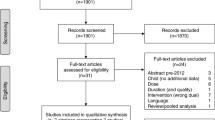
A total of 21,554 abstracts were identified through the systematic literature search. From the 604 full-text publications included in the broader scope review, 28 publications describing 16 RCTs were selected for inclusion [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Throughout this process, the identification of eligible trials followed the PICOS design criteria outlined in Supporting Information 1. The database search for relevant studies is outlined in Supporting Information 2. The flow of information diagram is presented in Fig. 1.
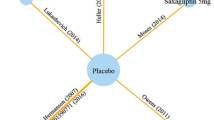
The complete network of evidence is shown in Fig. 2 and networks are presented by clinical outcome in Supporting Information 3. In summary, two studies assessed liraglutide [20, 32], four studies assessed empagliflozin [28,29,30, 33], seven studies assessed canagliflozin [23,24,25,26,27, 34, 35], three studies assessed dapagliflozin [21, 22, 31], and four studies assessed sitagliptin [20, 23, 24, 35]. Sitagliptin was included in the network to provide indirect evidence to the comparison of liraglutide with SGLT-2 inhibitors.
Studies were generally well matched in terms of baseline age, percentage of female patients, weight, and baseline HbA1c (Table 1). For HbA1c, however, the average trial baseline values in the SGLT-2 trials spanned from 7.2% to 8.1%, whereas the average trial baseline value was 8.4% in both liraglutide trials. Mean duration of diabetes varied from 4 to 9.7 years. Most studies incorporated treatment durations of approximately 26 weeks, though there was some deviation, with two studies having treatment durations of 12 weeks [23, 31] or 52 weeks [27, 35]. With respect to inclusion of 12-week studies, the NMA focuses on comparative effects between interventions, rather than absolute reductions. Thus, while stability of the treatment is generally expected to be reached between 12 and 24 weeks, little to no confounding by differences in time points is expected on comparative effects. Further, additional evidence comes with the advantage of strengthening the evidence base. Change from baseline for each outcome, as reported in each RCT, is presented in Supporting Information 4.
Network Meta-Analyses
A random-effects NMA model was used to estimate outcomes for change in HbA1c, proportion of patients achieving HbA1c targets, mean change in FPG, and mean change in weight as the DIC suggested a better model fit. A fixed-effects NMA was found to be more appropriate for estimating major and mild hypoglycemia outcomes, as results could not reach MCMC convergence under the random-effects model. The results of the edge-splitting exercise, which was used to compare outcomes as determined by indirect and direct evidence separately, are presented in Supporting Information 5.
HbA1c
Table 2 presents mean differences in CFB in HbA1c (%). All interventions were statistically superior to placebo, with liraglutide 1.2 and 1.8 mg offering the largest reductions compared to placebo (MD −1.02%, 95% CrI −1.28 to −0.73, and MD −1.18%, 95% CrI −1.45 to −0.89, respectively). Both doses of liraglutide were also statistically superior to all other doses of SGLT-2s, with the exception of the comparison between liraglutide 1.2 mg and canagliflozin 300 mg. Both doses of liraglutide were also found to be statistically superior to sitagliptin 100 mg in terms of HbA1c reduction.
The expected changes in HbA1c for each active intervention were modeled by combining the average placebo response with the relative treatment effect estimates of each treatment versus placebo (Fig. 3). However, as each modeled response also includes uncertainty in the average placebo response, this figure should not be used to make statistical comparisons between treatments.
HbA1c Target
The odds ratios of achieving target HbA1c levels (<7% or ≤7%, depending on the target defined in the respective RCT) are presented in Table 3. All treatments, with the exception of dapagliflozin 5 mg, were found to be statistically more efficacious than placebo in achieving HbA1c targets. Liraglutide 1.8 mg provided the highest odds of achieving target HbA1c compared to placebo (OR 9.80, 95% CrI 5.61–16.65) followed by liraglutide 1.2 mg (OR 6.51, 95% CrI 3.67–10.99). Liraglutide 1.8 mg was statistically superior to any other treatment. Similar trends were observed for liraglutide 1.2 mg, although no statistical superiority could be asserted for the comparisons to both empagliflozin doses.
FPG
Table 4 presents mean differences in CFB in FPG (mmol/dL) for each comparison in the network. All interventions in the network performed statistically superiorly to placebo in lowering FPG, with the greatest reduction seen with liraglutide 1.8 mg (MD −2.20 mmol/dL, 95% CrI −2.63 to −1.77). Liraglutide statistically lowered FPG compared to both doses of dapagliflozin. No statistical differences were observed when comparing high to high and low to low doses of liraglutide, canagliflozin, and empagliflozin.
Weight
Mean differences in CFB in weight are presented in Table 5. Liraglutide and all SGLT-2 inhibitors were associated with weight reductions that were statistically superior to placebo. Liraglutide generally appeared statistically comparable to all SGLT-2 inhibitors, and statistically superior to sitagliptin, albeit canagliflozin 300 mg was statistically superior to liraglutide 1.2 mg.
Hypoglycemic Events
Both major and minor hypoglycemic events were considered separately in the NMA and no differences were observed between the treatments for which data were available. For major hypoglycemic events, data were available for liraglutide, canagliflozin, dapagliflozin, empagliflozin, and sitagliptin. The available data for minor hypoglycemic events was more limited, with outcomes only available for liraglutide and dapagliflozin. The results of these analyses are summarized in Supporting Information 6; no statistically meaningful differences were found between any of the considered interventions.
Discussion
The purpose of this NMA was to compare the efficacy of liraglutide to SGLT-2 inhibitors among people with inadequately controlled T2DM despite treatment with metformin (alone or in combination with other OADs). Across all outcomes evaluated in this analysis, liraglutide performed at least as well as SGLT-2 inhibitors. Management with liraglutide was found to present larger reductions in CFB in HbA1c or FPG, and reaching HbA1c targets (<7% or ≤7%). Differences between treatments based on these outcomes were particularly marked for comparisons to liraglutide 1.8 mg. Moreover, liraglutide 1.8 mg was generally associated with more favorable outcomes. Few differences between liraglutide and SGLT-2 inhibitors in CFB in weight were observed. This finding suggests that there are no weight-related consequences associated with the use of liraglutide over the use of other T2DM treatments included in our analysis. Overall, the analyses indicated better efficacy outcomes with liraglutide.
No differences were observed in terms of hypoglycemia. However, this outcome must be interpreted with caution, given the low numbers of hypoglycemic events observed in the included trials and, in the case of minor hypoglycemia, the limited number of interventions that were available for inclusion in the network.
The population included people previously treated with metformin monotherapy as well as those on metformin in combination with other OADs, including SUs, TZDs, and DPP-4s. The nature of the evidence base did not allow for separate NMAs for these subpopulations. As it is possible that the number of prior OADs is an effect modifier, we cannot ignore the potential of this factor to impart bias or inconsistency into the model. To this end, some differences were observed across trials for baseline disease duration and HbA1c. For the latter, the two included liraglutide studies have slightly higher baseline HbA1c values compared to the SGLT-2 trials. Future studies may also evaluate the effects of differences in baseline HbA1c as well as the duration of treatment. While we did not observe strong evidence of inconsistency between direct and indirect comparisons of relative treatment effects in this population, future analyses may re-evaluate the evidence base to assess the impact of these factors.
In conclusion, liraglutide appears to offer better glycemic control in terms of lowering HbA1c levels, FPG, and achieving HbA1c targets in comparison to SGLT-2 inhibitors. Further, no weight-related consequences associated with the use of liraglutide over the use of SGLT-2s were observed. No differences were observed between treatments for hypoglycemia events, though this outcome must be interpreted with caution given the limitations of the available data. Overall, liraglutide has been demonstrated as an efficacious treatment option compared to SGLT-2 inhibitors in the management of people with T2DM on background OADs.
References
Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocrine Pract. 2016;22:84–113. doi:10.4158/ep151126.cs.
American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care. 2016;39(Suppl 1):S52–59. doi:10.2337/dc16-S010.
Robinson LE, Holt TA, Rees K, et al. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3. doi:10.1136/bmjopen-2012-001986.
Scott DA, Boye KS, Timlin L, et al. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab. 2013;15:213–23. doi:10.1111/dom.12007.
Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011:Cd006423. doi:10.1002/14651858.CD006423.pub2.
Gross JL, Kramer CK, Leitao CB, et al. Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med. 2011;154:672–9. doi:10.7326/0003-4819-154-10-201105170-00007.
McIntosh B, Cameron C, Singh SR, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5:e35–48.
Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi:10.1007/s40265-014-0337-y.
Abdul-Ghani MA, Norton L, DeFronzo RA. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr Diab Rep. 2012;12:230–8. doi:10.1007/s11892-012-0275-6.
Orme M, Fenici P, Lomon ID, et al. A systematic review and mixed-treatment comparison of dapagliflozin with existing anti-diabetes treatments for those with type 2 diabetes mellitus inadequately controlled by sulfonylurea monotherapy. Diabetol Metabol Syndr. 2014;6:73. doi:10.1186/1758-5996-6-73.
Goring S, Hawkins N, Wygant G, et al. Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes Obes Metab. 2014;16:433–42. doi:10.1111/dom.12239.
Liakos A, Karagiannis T, Bekiari E, et al. Update on long-term efficacy and safety of dapagliflozin in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metabol. 2015;6:61–7. doi:10.1177/2042018814560735.
White JR Jr. Empagliflozin, an SGLT2 inhibitor for the treatment of type 2 diabetes mellitus: a review of the evidence. Ann Pharmacother. 2015;49:582–98. doi:10.1177/1060028015573564.
Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24. doi:10.1002/sim.1875.
Sutton A, Ades AE, Cooper N, et al. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26:753–67.
Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–44. doi:10.1002/sim.3767.
Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91.
Spiegelhalter D, Best N, Carlin B, et al. Bayesian measures of model complexity and fit (with discussion). J R Stat Soc B. 2002;64:583–639.
Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56. doi:10.1016/s0140-6736(10)60307-8.
Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–33. doi:10.1016/s0140-6736(10)60407-2.
Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metabol. 2012;97:1020–31. doi:10.1210/jc.2011-2260.
Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–8. doi:10.2337/dc11-1926.
Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–92. doi:10.1007/s00125-013-3039-1.
Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–77. doi:10.1111/dom.12273.
Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–82. doi:10.1111/ijcp.12322.
Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50. doi:10.1016/s0140-6736(13)60683-2.
Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9. doi:10.2337/dc13-2105.
Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–404. doi:10.2337/dc12-2673.
Kovacs C, Seshiah V, Swallow R, et al. Empagliflozin as add-on to pioglitazone with or without metformin improves glycemic control in patients with type 2 diabetes (T2DM). Diabetes. 2013;62:A291. doi:10.2337/db13-859-1394.
Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62. doi:10.1111/dom.12127.
Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi:10.2337/dc08-1355.
Patel S, DeFronzo R, Lewin A, et al. Fixed dose combinations of empagliflozin/linagliptin for 52 weeks as add-on to metformin in subjects with type 2 diabetes. Diabetologia. 2014;(1):S7. doi:10.1007/s00125-014-3355-0.
Qiu R, Capuano G, Meininger G. Efficacy and safety of twice-daily treatment with canagliflozin, a sodium glucose co-transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2014;1:54–60. doi:10.1016/j.jcte.2014.04.001.
Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–15. doi:10.2337/dc12-2491.
Acknowledgements
This study and article processing charges were sponsored by Novo Nordisk. The sponsor contributed to the design of the study, but did not have a role in the analysis or interpretation. The manuscript was drafted by Precision Health Economics and approved by the sponsor. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All data generated or analyzed during this study are included in this published article/as supplementary information files.
Disclosures
Uffe Plough is an employee and shareholder of Novo Nordisk AS. Jakob Langer is an employee and shareholder of Novo Nordisk AS. Rasmus Skovgaard is an employee and shareholder of Novo Nordisk AS. Maria Lorenzi is an employee of Precision Health Economics. Michael Zoratti is an employee of Precision Health Economics. Jeroen Jansen is an employee of Precision Health Economics. Precision Health Economics is a health economics and analytics company commissioned by Novo Nordisk to conduct the presented systematic review and network meta-analysis and prepare the manuscript.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D137F0600D7FB925.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lorenzi, M., Ploug, U.J., Langer, J. et al. Liraglutide Versus SGLT-2 Inhibitors in People with Type 2 Diabetes: A Network Meta-Analysis. Diabetes Ther 8, 85–99 (2017). https://doi.org/10.1007/s13300-016-0217-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0217-4