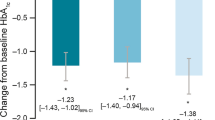Abstract
Introduction
The First Basal Insulin Evaluation (FINE) Asia study was a prospective, observational registry evaluating basal insulin initiation in Asian patients with type 2 diabetes mellitus inadequately controlled by oral antihyperglycemic agents.
Methods
The objective of this post hoc analysis was to observe and report the findings from individual participating countries. The primary endpoint was change in glycosylated hemoglobin (HbA1c) from baseline to month 6 after basal insulin initiation. Secondary endpoints included change in fasting blood glucose (FBG), percent of patients achieving target HbA1c and FBG levels, average insulin doses, and hypoglycemic events.
Results
The study included 2921 patients from 11 Asian countries at baseline, 2679 (92%) of whom had evaluable data. Following initiation of basal insulin (neutral protamine Hagedorn insulin, glargine, or detemir), there was a significant (P < 0.001) difference in HbA1c reduction and proportions of patients meeting HbA1c and FBG targets (<7% and <110 mg/dL, respectively) across all country cohorts by month 6. Glycemic control also varied greatly, with 7.4% (Taiwan) to 71.5% (China) of patients reaching target HbA1c <7% levels. Mean (±standard deviation) insulin dose increases over the 6-month period ranged from 0.5 ± 3.1 U (Pakistan) to 6.0 ± 8.6 U (Thailand). Hypoglycemia rates also varied, with 7.1% (India) to 27.3% (China) of patients experiencing one or more events.
Conclusions
Data from the FINE-Asia registry study show widely varying degrees of baseline comorbidities and glycemic control in patients among the country cohorts observed. Countries with >9 years of diabetes prior to insulin initiation had the lowest reductions in HbA1c and proportions of patients achieving HbA1c and FBG targets, suggesting that earlier basal insulin initiation may afford better glycemic control in these patients.
Funding
This study was funded by Sanofi.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by insulin resistance and decline of β-cell function [1, 2]. Tight glycemic control is one of the cornerstones of effective management of T2DM, as it significantly reduces the risk of microvascular complications and may reduce the impact of macrovascular problems, particularly when achieved early in the disease course [3–5].
Based on clinical and experimental studies, the International Diabetes Federation proposed a preferred glycosylated hemoglobin (HbA1c) target of ≤6.5% for the management of T2DM [3–6]; experts from the American Diabetes Association and the European Association for the Study of Diabetes issued a statement advocating HbA1c levels of <7.0%, with stringency of control adapted according to patient-specific features such as age, disease duration, and comorbidities, among others [7]. This statement recognized that insulin can be an effective component of management strategies to achieve glycemic control, and early initiation of insulin is recommended in patients not meeting HbA1c targets. The benefits of insulin therapy and the recommendations for its initiation in treatment guidelines have been demonstrated via observational studies and medical insurance database analyses conducted in European and North American countries [8–10]. The translation of this to the Asian experience, however, has not specifically been reported. At the same time, the prevalence of T2DM continues to increase at higher rates among Asian countries than in other regions [11].
Available evidence suggests that insulin utilization in Asia has not markedly changed over the past 10 years [12–14], despite changes in treatment guidelines advocating the initiation and intensification of therapy to reach HbA1c goals. The objective of the First Basal Insulin Evaluation (FINE)-Asia study was to provide details on the real-world initial insulinization in patients with T2DM across Asia, as well as to determine the tolerability and efficacy of basal insulin regimens in this region. This article provides information from post hoc analysis of the FINE-Asia study, which examined variations in baseline characteristics and efficacy and safety endpoints according to the country or region from which patients were enrolled.
Methods
FINE-Asia was a multinational, prospective, observational study designed to assess the initiation of basal insulin in insulin-naïve patients with T2DM in a real-world clinical setting in Asia. Patients were enrolled from 195 centers/sites across 11 different Asian countries (Bangladesh, China, Hong Kong, India, Indonesia, Korea, Pakistan, Singapore, Taiwan, Thailand, and Vietnam) [15].
Study Objectives
The FINE-Asia study was designed to evaluate current clinical practice in the treatment of Asian patients with T2DM with inadequate glycemic control on oral antidiabetic drugs (OADs) who have been initiated on basal insulin. The main objective of this post hoc analysis was to examine the baseline characteristics of the study population and to report the findings of the study according to the country or region from which patients were enrolled. The primary efficacy endpoint was the change in HbA1c from baseline to month 6 after basal insulin initiation. Secondary endpoints included change in fasting blood glucose (FBG) from baseline to month 6, percent of patients achieving target HbA1c and FBG levels, average insulin doses, and hypoglycemic events.
Patients
From the centers/sites participating in this registry study, male or female patients aged ≥20 years with T2DM inadequately controlled (HbA1c ≥ 8%) by OADs, and who, in the opinion of their treating physician, required the initiation of a basal insulin, were eligible for inclusion in the registry.
Patients who received premixed insulin at the start of the registry period, who were prescribed insulin shortly before the start of the registry period (with the exception of acute rescue insulin therapy), or women who were either pregnant or of childbearing potential (not surgically sterile or postmenopausal for less than 2 years) and were not going to use a reliable contraceptive measure for the duration of the study were not included in this registry. In addition, patients with known hypersensitivity to insulin or any excipients of marketed insulin were also excluded from the study.
Study Treatment and Assessments
Basal insulin was initiated with or without concomitant OADs, and no specific protocol on the type of basal insulin or OADs administered was recommended. The doses of basal insulin were based on the recommendation of locally approved package inserts and individually adjusted by the treating physicians based on individual patient profiles (e.g., comorbidities, tolerability/preference, etc.). Follow-up duration was 6 months; the registry involved three main visits scheduled according to physician routine practice, at which point effectiveness and safety data were collected (at inclusion, month 3 ± 7 days, and month 6 ± 7 days). Each visit included standard physical examinations (including body weight and blood pressure) and assessments of HbA1c, FBG and self-monitoring of blood glucose (SMBG) profiles, adverse drug reactions (ADRs), and hypoglycemic episodes. It was recommended that patients perform SMBG using their own glucose monitors by their usual practice. In addition, SMBG was recommended when mild to moderate hypoglycemic events occurred. Safety was evaluated using the ADRs reported during the follow-up period, including all non-serious ADRs (especially hypoglycemic events), serious ADRs, overdoses, and changes in clinical and/or laboratory data. Severe hypoglycemia was defined as blood glucose (BG) <70 mg/dL (3.9 mmol/L) and requiring assistance.
Statistics
Statistical analyses were based on patients with HbA1c data at both baseline and 6 months. All data were analyzed using SAS version 9.2 statistical software (SAS institute, Cary, NC) and summary statistics (mean, median, range, and standard deviation for continuous variables, and number and percent for categorical variables) were determined. Student’s paired t test was used to compare parameters before and after the treatment period, and qualitative variables and between-group comparisons were analyzed using Fisher’s exact probability test or chi-square tests as appropriate. All statistical tests were performed using two-tailed tests at a 5% level of significance.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
Baseline Characteristics of the Study Population
The study included 2921 patients from 11 Asian countries at baseline; 2679 patients with both baseline and 6-month HbA1c values were included in this post hoc analysis. Demographic characteristics and insulin regimen at baseline are shown in Table 1. Most patients initiated insulin therapy with insulin glargine. Mean (±standard deviation) insulin dose at baseline ranged from 9.5 ± 3.4 U/day in Thailand to 15.2 ± 6.3 U/day in Taiwan. Across the countries/region cohorts examined, mean duration of diabetes ranged from 6.3 ± 5.2 years (China) to 11.5 ± 7.1 years (Taiwan), and baseline HbA1c ranged from 9.4% ± 1.2% (India) to 10.5 ± 1.9% (SE Asia). Mean baseline HbA1c (10.5 ± 1.9%) and FBG (230 ± 69.0 mg/dL [12.8 ± 3.8 mmol/L]) were highest in patients from South-East Asia (group defined in this study as Bangladesh, Hong Kong, Indonesia, Singapore, and Vietnam). Patients from Taiwan had the longest duration of OAD use (11.1 ± 7.0 years; primarily sulfonylurea and/or biguanides) and were among those with the highest baseline HbA1c level (10.2 ± 1.7%). Patients from China had the shortest duration of OAD use (5.8 ± 5.2 years) and were among those with the lowest baseline HbA1c (9.4 ± 1.6%) and FBG (185 ± 47.2 mg/dL [10.3 ± 2.6 mmol/L]) levels. In contrast to patients from SE Asia (24.2 ± 3.8 kg/m2), patients from Pakistan (27.9 ± 6.3 kg/m2) had the highest body mass index. The prevalence of diabetic neuropathy or nephropathy ranged from 14.1% (SE Asia) to 39.2% (India), coronary artery disease from 7.0% (Pakistan) to 21.4% (China), and dyslipidemia from 48.8% (China) to 84.4% (Thailand) (Table 2).
Efficacy and Safety by Country at 6 Months
After the addition of basal insulin therapy, significant decreases in HbA1c (P < 0.001) were observed from baseline to month 6 for each individual country/region, with decreases differing significantly between country cohorts (P < 0.001), ranging from −1.3% in Taiwan to −2.6% in China and Pakistan (Fig. 1; Table 3). After basal insulin initiation, significant reductions in FBG for each individual country cohort were also observed (P < 0.001). In addition, increases in the proportion of patients achieving FBG < 110 mg/dL (6.1 mmol/L) were observed at 6 months, although these proportions varied significantly (P < 0.001 between each country/region). Proportions (at 6 months) ranged from 24.7 to 27.6% among the patients in Taiwan, Korea, South-East Asia, and Thailand, and from 43.7 to 64.8% among those in India, China, and Pakistan (Fig. 2).
Wide variations between the cohorts in individual countries/regions were also observed in terms of the proportion of patients achieving HbA1c < 7% at 6 months, with percentages ranging from 7.4% (Taiwan) to 71.5% (China) (Table 3). These variations were also observed with respect to mean insulin dosing, where mean daily dose increases ranged from 0.5 ± 3.1 U/day for the patients in Pakistan to 6.0 ± 8.6 U/day in the Thailand cohort (Table 3).
After basal insulin initiation, significant differences were observed in the proportion of patients experiencing at least one hypoglycemic event during the 6 months of the study (P < 0.001), with the Indian cohort having the lowest percentage (7.1%) and the Chinese cohort the highest (27.3%).
Discussion
The Asia–Pacific region comprises more than half of the world’s population and has the largest diabetes burden in the world, with hypertension, dyslipidemia, and metabolic syndrome also highly prevalent [16]. India, China, and Pakistan make up three of the top 10 countries having the most people with diabetes, while the prevalence of T2DM in Thailand is 9.8%—double the number forecast by the World Health Organization [17, 18]. An increased risk of death associated with high HbA1c, age, history of coronary artery disease, and cerebrovascular disease has been reported from the Thailand Diabetic Registry cohort [18]. As a result, establishing HbA1c goals and treating patients in these countries, with the intent of rapidly and effectively achieving target HbA1c levels, is crucial to successful glycemic control.
Data from this FINE-Asia study show widely varying degrees of glycemic control in patients with T2DM, depending on country of residence. Of the country cohorts observed, the highest percentage of patients reaching target HbA1c levels of <7.0% at study end (71.5%) was observed in Chinese patients. Along with India, China had the joint lowest mean HbA1c levels (9.4%) at baseline, yet only 43.0% of Indian patients with T2DM reached target HbA1c levels of <7.0% at study end. It should be noted that Chinese patients had the shortest duration of diabetes (6.3 years) and OAD use (5.8 years) of any country studied, including India. In line with findings from previous studies [19], as well as the Chinese Guidelines for Prevention and Treatment of Diabetes, Chinese patients with T2DM appear to benefit from earlier initiation of treatment. Rates of hypoglycemia, however, were highest (27.3%) in Chinese patients and lowest among those in India (7.1%). Although hypoglycemia is a well-reported side effect of insulin treatment [20], these findings highlight the importance of monitoring glucose levels upon initiation of basal insulin treatment, particularly when an early, aggressive approach to glycemic control is undertaken. While the reasoning for the disparity in hypoglycemia rates cannot be fully elucidated, duration of diabetes (6.3 vs. 9.8 years among the Chinese versus Indian cohort, respectively), duration of OAD usage (5.8 vs. 9.2 years), and neutral protamine Hagedorn usage (37.5% vs. 5.0% of patients), among other characteristics, are under consideration for further analysis.
Study cohorts from Korea and Taiwan had the longest duration of diabetes (10.7 and 11.5 years, respectively), as well as OAD use duration (9.2 and 11.1 years); baseline HbA1c levels were also among the highest of all the countries analyzed. Interestingly, both countries had the smallest change in HbA1c and the lowest proportion of patients achieving HbA1c and FBG goals at 6 months. In these countries, patients did not receive insulin treatment until much later in the course of their disease, suggesting that earlier initiation of insulin treatment may result in more favorable glycemic control.
The concept of real-world clinical practice observational studies provides an expanded opportunity to observe and analyze therapeutic management strategies outside of more rigid, protocol-driven controlled clinical trials [21]. This is particularly important in diabetes, where both physician- and patient-based decisions and practice can impact outcomes. Multinational observational studies such as A1chieve (NCT00869908) [22], IMPROVE (NCT00659282) [23], PREDICTIVE (NCT00659295) [24], and PRESENT [25], among others, have utilized real-world clinical practice as the basis for observing and recommending management approach adjustments to optimize glycemic control in the context of insulin initiation. Many of these studies, such as A1chieve and CREDIT [26], are longer in duration and aim to analyze multifactorial aspects of long-term impact of treatments and treatment strategies. Our study, not unlike other studies mentioned above, was limited to 6 months’ duration. This was due to the fact that the study goal was to observe potential effects of insulin initiation; longer duration of observation, while informative, would potentially risk confounding the intent of the study with longer-term management adjustments.
As with the aforementioned observational studies, there are limitations and considerations that should be acknowledged. Lack of randomization, predefined visits, or protocol-driven care could potentially result in variations between patients and countries with respect to individual diabetes management.
Varied clinical practice between countries should be considered when interpreting these data. For example, in some Asian countries it is not unusual for physicians to discontinue OAD treatment when initiating basal insulin, either for cost- or patient-related reasons; as such, this may have influenced between-country differences in glycemic control. In general, country-specific approaches to insulin initiation (initial dosing, patient education, dietary behavior, procedural variation in management), as well as choice of OAD, may influence clinical response, yet it is important to note that this is reflective of real-world clinical practice, which was a key aspect of our study. There is a lack of available information regarding time of insulin administration, SMBG, and concomitant medications, which also may influence glycemic control. Since HbA1c, FBG, and other clinical measurements were performed in different laboratories/hospitals and outside a rigid, protocol-driven setting, inconsistencies/errors may be possible.
Glycemic management differences between countries were directly observed, and other country-based factors likely warrant consideration as well. When considering basal insulin choice, no patients in any of the other participating countries (with the exception of 0.7% of patients in India) were using insulin detemir, except for 13.4% of patients in Taiwan. Findings of recently published studies [27–29] suggest that the use of insulin glargine is associated with greater glycemic control than insulin detemir at the same dose.
The study populations observed herein represent a subset of patients with unacceptably poor glycemic control (mean HbA1c ranging from 9.4% to 10.5%). These HbA1c levels would intuitively result in more substantial reductions in the glycemic endpoints observed in our study compared with randomized clinical trials; nevertheless, the HbA1c reductions observed in our study (–1.27% to 2.64%) are not drastically higher than those seen in the overall FINE-Asia population, in other multinational observational studies [8], or in randomized controlled trials in patients initiating basal insulin [30, 31].
The results of this prospective, observational, registry-based study in these Asian countries suggest that initiation of insulin therapy is prolonged considerably in many Asian countries, and that glycemic control remains suboptimal in many patients. Given the importance of T2DM to the health care of the region, this is a notable concern. Duration of diabetes prior to insulinization, comorbidities, as well as baseline insulin choice and dosing (both at initiation and throughout treatment), varied significantly between countries, all of which may have impacted the level of glycemic control achieved and should be studied further.
Conclusion
Importantly enough, these data confirm that local conditions—as reflected by the country-based outcomes observed herein—may play an important role in the pattern of care and outcomes. Ultimately, these country-specific findings support those from the overall FINE-Asia study, suggesting that in Asian patients with T2DM, initiation of basal insulin earlier in the course of diabetes treatment may be considered a factor for better glycemic control.
References
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37–42.
Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–9.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
International Diabetes Federation. IDF Clinical Guidelines Task Force. Global guideline for Type 2 diabetes. International Diabetes Federation. http://www.idf.org/global-guideline-type-2-diabetes-2005. Accessed 3 Dec 2013.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Assocation and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Liebl A, Breitscheidel L, Nicolay C, Happich M. Direct costs and health-related resource utilisation in the 6 months after insulin initiation in German patients with type 2 diabetes mellitus in 2006: INSTIGATE study. Curr Med Res Opin. 2008;24:2349–58.
Nichols GA, Gandra SR, Chiou CF, Anthony MS, Alexander-Bridges M, Brown JB. Successes and challenges of insulin therapy for type 2 diabetes in a managed-care setting. Curr Med Res Opin. 2010;26:9–15.
Karter AJ, Moffet HH, Liu J, et al. Glycemic response to newly initiated diabetes therapies. Am J Manag Care. 2007;13:598–606.
International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium. International Diabetes Federation. http://www.idf.org/diabetesatlas. Accessed 3 Dec 2013.
Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care. 2009;32:227–33.
Mohamed M. An audit on diabetes management in Asian patients treated by specialists: the Diabcare-Asia 1998 and 2003 studies. Curr Med Res Opin. 2008;24:507–14.
Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–9.
Tsai ST, Pathan F, Ji L, et al. First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J Diabetes. 2011;3:208–16.
Tan DA. Changing disease trends in the Asia–Pacific. Climacteric. 2011;14:529–34.
Ali MK, Narayan KM, Tandon N. Diabetes & coronary heart disease: current perspectives. Indian J Med Res. 2010;132:584–97.
Pratipanawatr T, Rawdaree P, Chetthakul T, et al. Thailand Diabetic Registry cohort: predicting death in Thai diabetic patients and causes of death. J Med Assoc Thai. 2010;93(Suppl 3):S12–20.
Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60.
Barendse S, Singh H, Frier BM, Speight J. The impact of hypoglycaemia on quality of life and related patient-reported outcomes in Type 2 diabetes: a narrative review. Diabet Med. 2012;29:293–302.
Ligthelm RJ, Borzi V, Gumprecht J, Kawamori R, Wenying Y, Valensi F. Importance of observational studies in clinical practice. Clin Ther. 2007;29:1284–92.
Home PD, El Naggar N, Khamseh M, Gonzalez-Galvez G, Shen C, et al. An observational, non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352–63.
Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, et al. The IMPROVE study: a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract. 2008;62:1809–19.
Meneghini LF, Donhorst A, Sreenan S, the PREDICTIVE Study Group. Once-daily insulin detemir in a cohort of insulin-naive patients with type 2 diabetes: a sub-analysis of the PREDICTIVE study. Curr Med Res Opin. 2009;25:1029–35.
Khutsoane D, Sharma SK, Almustafa M, Jang HC, Azar ST, et al. Biphasic insulin aspart 30 treatment improves glycemic control in patients with type 2 diabetes in a clinical practice setting. Diabetes Obes Metab. 2008;10:212–22.
Home PD, Dain MP, Freemantle N, Kawamori R, Pfohl M, et al. Four-year evolution of insulin regimens, glycaemic control, hypoglycaemia and body weight after starting insulin therapy in type 2 diabetes across three continents. Diabetes Res Clin Pract. 2015;108:350–9.
Eland I, Heintjes E, Houweling L, deGrooth R, Veneman TF, Bouter KP. Insulin glargine versus insulin detemir: glycemic control and insulin dose in type 2 diabetes mellitus patients using a medical record linkage system in The Netherlands. J Diabetes Metab. 2011;2:165.
Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–16.
Swinnen SG, Dain MP, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care. 2010;33:1176–8.
Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care. 2009;32:1007–13.
Owens DR, Traylor L, Dain MP, Landgraf W. Efficacy and safety of basal insulin glargine 12 and 24 weeks after initiation in persons with type 2 diabetes: a pooled analysis of data from treatment arms of 15 treat-to-target randomised controlled trials. Diabetes Res Clin Pract. 2015;106:264–74.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Sanofi. Editorial support was provided by Albert M. Balkiewicz, MSc (PPSI, Hackensack, NJ, USA), and Leigh Prevost, MSc, (PPSI, Worthing, UK), and was funded by Sanofi. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Linong Ji has no conflicts of interest to disclose.
Shih-Tzer Tsai has been a consultant for Sanofi, Takeda, and Eli Lilly, and has received honoraria for speaker services from Sanofi and Novo Nordisk.
Jay Lin is a consultant for Sanofi.
Sanjiv Bhambani has been a consultant for and received honoraria from Sanofi, Merck Sharpe & Dohme, Bristol-Myers Squibb, and Novartis.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ji, L., Tsai, ST., Lin, J. et al. National Variations in Comorbidities, Glycosylated Hemoglobin Reduction, and Insulin Dosage in Asian Patients with Type 2 Diabetes: The FINE-Asia Registry. Diabetes Ther 6, 519–530 (2015). https://doi.org/10.1007/s13300-015-0137-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-015-0137-8