Abstract
Objectives
Metformin is an established first-line treatment for type 2 diabetes mellitus (T2DM) patients but intensification of oral anti-diabetic therapy is usually required over time. The effectiveness of diabetes control with vildaGliptin and vildagliptin/mEtformin (EDGE) study compared effectiveness and safety of vildagliptin and other oral anti-diabetic drugs (OAD) in 45,868 patients worldwide with inadequately controlled T2DM by monotherapy under real-life conditions. Here, we present effectiveness results for patients receiving vildagliptin (vildagliptin cohort) or another OAD (comparator cohort) add-on to monotherapy in Bulgaria.
Methods
The eligible diabetes patients inadequately controlled with current monotherapy were assigned to add-on treatment, which was chosen by the physician based on patient’s need. Effectiveness was assessed by glycated hemoglobin (HbA1c) drop and by means of a composite endpoint assessing the proportion of patients responding to treatment (HbA1c <7%) without proven hypoglycemic event and significant weight gain (>5%) after 12 months of treatment.
Results
In total, 754 patients were enrolled in Bulgaria, 384 in the vildagliptin cohort and 369 in the comparator cohort. Mean HbA1c change from baseline was significantly higher with vildagliptin compared to the comparator (−1.35% in the vildagliptin cohort and −0.55% in the comparator cohort, P < 0.001). In the vildagliptin cohort, a higher proportion of patients reached the composite endpoint (HbA1c <7%, no hypoglycemic events, no weight gain) when compared to the comparator cohort (vildagliptin: 32.3%; comparator: 8.4%; P < 0.001). Overall, vildagliptin was well tolerated with similarly low incidences of total adverse events (3.4% versus 1.9% in the comparator group) and serious adverse events (2.3% versus 1.1% in the comparator group).
Conclusions
In real-life clinical practice in Bulgaria, vildagliptin is associated with a greater HbA1c drop, and a higher proportion of patients reaching target HbA1c without hypoglycemia and weight gain compared to comparator.
Similar content being viewed by others
Introduction
Almost half of patients with type 2 diabetes mellitus (T2DM) do not achieve globally recognized blood glucose targets [1, 2]. It is difficult to understand the reasons for this, considering that during last decades the armamentarium of resources to treat T2DM has significantly increased. The reasons can be sought in two main directions.
The first trend observed is the dramatic increase in global prevalence of this disease according to the International Diabetes Federation. Currently, the prevalence in the age group 20–79 years is 8.3% (382 million people worldwide), but it is expected to rise with 55% to a prevalence of 10.1% (592 million) by 2035 [3]. For Bulgaria, the number of patients with T2DM in 2013 was 427,000 people in the age group 20–79 years (7.6%) according to the same source. It is expected the prevalence of diabetes in Bulgaria will follow the global trends. The enormous financial resources destined to the treatment of diabetic complications are constantly increasing requiring identification of new therapeutic approaches to delay complications in time.
The second trend observed is the medications available for T2DM, until recently, do not completely address all main pathogenic mechanisms of the disease. It is well known that T2DM is a chronic disease that results from a combination of insulin resistance and insulin deficiency caused by beta-cell dysfunction [4]. The progressive nature of the disease requires effective glycemic control to reduce the risk of long-term micro- and macrovascular complications related to dysglycemia [5]. Metformin is the most widely used oral anti-diabetic drug (OAD) and is recommended as first-line therapy for patients with T2DM [5]. However, as glycemic control deteriorates, patients with T2DM usually require more than one antidiabetic agent to achieve glycemic targets [6–8]. Sulfonylureas (SUs) are one of the most commonly used second-line treatment options of T2DM [5] in clinical practice usually in combination with metformin [9]. SUs are commonly associated with weight gain and hypoglycemia [5, 10]. In patients with T2DM receiving OADs, both weight gain and hypoglycemia are independently associated with lower treatment satisfaction and lower health-related quality of life [11]. These adverse events are a well-recognized reason for poor adherence to chronic therapy, which finally results in impaired disease control [12–14].
The new therapeutic classes such as incretin-based therapies could be a solution for some of the problems faced in treatment of T2DM, such as improvement of glycemic control for longer periods, limitation of glycemic fluctuations, hypoglycemia, and weight gain. Long-term effectiveness and safety trials are ongoing to investigate the potential of their new mode of action to overcome the burden of diabetes, to improve diabetes control and to eliminate long-term complications, with special focus on cardiovascular outcomes [15].
International guidelines [5, 16] for the treatment of T2DM underwent several modifications in the last decade due to the fast dynamics of the understanding of the disease pathogenesis and the introduction of corresponding new medicines. This was based on numerous randomized controlled clinical trials (RCT), including ACCORD [17], ADVANCE [18], VADT [19], that were conducted and provided arguments of highest level for the evidence-based medicine. Despite RCTs high informative value, these studies have often been blamed for lack of generalizability of their findings because of the precisely enrolled subjects, better therapy compliance, medicines variations and strictly regulated dose regimen. After all, this is not the usual population, and it receives a strictly pre-determined treatment, beyond the usual practice. For all these, RCTs do not correspond to the routine practice and therefore a new type of pragmatic trial needs to be conducted in real-life settings, which will not replace RCTs, but will rather provide additional information and will help build a uniform concept of the treatment of diabetes mellitus [18, 20–22]. The number of these real-life clinical trials is still too small.
As mentioned above, metformin is used as a therapy of choice for the treatment of T2DM, irrespective of patient’s body weight, when adequate results cannot be achieved by diet and physical activity alone [5, 16]. The latest clinical recommendations of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) from 2012 [5], and of the American Association of Clinical Endocrinologists (AACE) from 2013 [16] suggest that the choice of a second agent to be added in case of inadequate efficiency of metformin monotherapy is based on individual judgment. It is important to underline that in these key strategic papers, the incretin-based therapy is constantly gaining positions, with the tendency to become second-line therapy added on the top of metformin.
A recent meta-analysis conducted to assess the efficacy and safety of all available second-line antihyperglycemic therapies in patients with T2DM inadequately controlled by metformin monotherapy revealed that dipeptidyl peptidase-4 (DPP-4) inhibitors are as effective as the other therapeutic classes in improvement of glycemic control, but with modest benefits with respect to weight gain and overall hypoglycemia [23].
Maintaining good glycemic control is essential for risk reduction of micro- and macrovascular complications associated with diabetes [24]. DPP-4 inhibitors possess a number of pharmacological attributes that would suggest cardiovascular safety. In addition to glucose lowering and weight neutrality, they lower blood pressure, improve postprandial hyperlipidaemia, reduce inflammatory markers, diminish oxidative stress, and improve endothelial function [25].
However, large-scale clinical trials, including studies from routine clinical practice, are also needed to identify benefits or harms from this therapy in a real-life setting. Such a study is the multinational study entitled effectiveness of diabetes control with vildaGliptin and vildagliptin/mEtformin (EDGE) with protocol number CLAF237A2404 [26].
Cardiovascular disease (CVD) is a major cause of morbidity and mortality for individuals with diabetes and the largest contributor to the direct and indirect costs of diabetes. The common conditions coexisting with type 2 diabetes (e.g., hypertension and dyslipidemia) are clear risk factors for CVD, and diabetes itself confers independent risk [27]. Bulgaria has one of the highest rates of premature death from cardiovascular disease in Europe [28] and despite clear recommendations in current guidelines for the treatment of T2DM (5, 16), ineffective monotherapy regimens remain high with many patients not receiving timely therapy intensification and consequently suffering with insufficient glycemic control [29]. The proportion of patients treated with novel therapeutic drug classes including DPP-4 inhibitors is relatively low. There is also an absence of depth analysis of the therapeutic behavior of physicians treating T2DM in Bulgaria and studies identifying the major problems facing in routine clinical practice. The aim of this sub-analysis was to assess effectiveness and tolerability of second-line vildagliptin combinations versus other OAD two-agent combinations for patients enrolled in Bulgaria.
Methods
Study Design
The EDGE was a 1-year, multinational, multicenter, post-authorization, prospective, observational study conducted in 45,868 subjects at 2,957 sites in 27 countries, grouped into 5 regions in which vildagliptin is approved: East Asia, Europe, Latin America, Near East, and India. Of those, 754 patients were enrolled from 24 sites in Bulgaria. The study included patients aged over 18 years, with T2DM and inadequate glycemic control while receiving OAD monotherapy with metformin, a SU, α-glucosidase inhibitor (AGI) or thiazolidinedione (TZD) and for whom, at the physician’s discretion a second OAD was considered. The physician could institute any medicine he/she might deem appropriate, except an incretin-based therapy [DPP-4 inhibitors/glucagon-like peptide-1 (GLP-1) agonists] other than vildagliptin. Patients who were planned to initiate a DPP-4 inhibitor other than vildagliptin, or an incretin mimetic/analog, or who required three or more OADs at study entry were excluded. Patients who were using insulin at the time of study entry and patients with a history of hypersensitivity to any of the study drugs or drugs of similar chemical classes were also excluded.
Patients were assigned by their physician to one of the following two groups: vildagliptin group (vildagliptin 50 mg twice daily add-on as second OADs or vildagliptin/metformin 50 mg/850 or 1,000 mg twice daily) or comparator group (add-on second OADs—SU, metformin, TZD, meglitinides, AGI to the ineffective monotherapy).
The present sub-analysis does not contain any new studies with human or animal subjects performed by any of the authors.
Data Collection
During the first routine visit the following patient baseline (BL) data were collected: age, gender, race, ethnicity, body weight, medical history, T2DM duration, T2DM therapy prior to study entry, newly initiated add-on OAD (second component of index medication), other medications (by class), most recent HbA1c test date and result, other laboratory tests. To estimate the glomerular filtration rate (GFR) by the modification of diet in renal disease (MDRD) method, creatinine data were collected. After 12 months, the final data collected included body weight, changes of index medication, most recent HbA1c test data and result, other laboratory test dates and results, adverse events (AEs) and serious AEs (SAEs) and study completion status.
Evaluation Criteria in EDGE Study
The primary effectiveness endpoint (PEP) was defined as the proportion of patients having a treatment response (HbA1c reduction from BL to Month 12 endpoint >0.3%) and no tolerability issues [peripheral edema, proven hypoglycemic event, discontinuation due to a gastrointestinal (GI) event, or weight gain ≥5%) [30]. As described in the primary manuscript regarding EDGE study results [26], this composite endpoint was chosen on the basis of the balanced decisions that clinicians need to make when choosing a glucose-lowering agent, namely the combination of efficacy [as defined by European Medicines Agency (EMA) and the Food and Drug Administration (FDA)] and most common side effects. For more details, please refer to original article [26].
Patients who could not be categorized as a success or failure (e.g., due to missing HbA1c or body weight data at 12-month endpoint) were considered non-evaluable. Non-evaluable patient data were considered failures in calculation of the odds ratio (OR) for success. The main analysis of the PEP utilized the per protocol (PP) population; data were censored if patient changed index therapy. Hypoglycemia was defined as symptoms suggestive of hypoglycemia that resolved promptly on the administration of oral carbohydrate (including mild and severe events). The most important endpoint from clinical perspective was the secondary effectiveness endpoints (SEP), defined as the proportion of patients with BL HbA1c ≥7% that achieved target HbA1c <7.0% after 12 months, without weight gain ≥3% or confirmed hypoglycemic episodes in subjects with baseline HbA1c ≥7%.
The secondary safety endpoints were determination of the time to death, SAE or AE occurring in the vildagliptin group [vildagliptin/metformin (fixed combinations)] versus the comparator group in real clinical practice settings; interruption of the treatment assigned due to a SAE; interruption of the treatment assigned due to an AE; interruption of the treatment assigned due to any cause other than SAE/AE; assessment of the effect of adding vildagliptin or vildagliptin/metformin (fixed combination) on the individual tolerability factors (body weight, peripheral edema, confirmed hypoglycemic episodes, and GI events) compared to the reference group in which another OAD is added.
Safety was assessed by AE reporting and measurement of specific laboratory values. Specific attention was given to hepatic safety due to a requirement for liver function monitoring prior and during treatment with vildagliptin [31].
All these combined endpoints were defined in agreement with the European Medicines Agency when this study was designed.
Statistical Analysis
This post hoc analysis provides mainly descriptive statistics. Inference is provided for primary and secondary endpoints. For these, the probability of success was analyzed using a binary logistic regression model to calculate ORs with 95% confidence intervals (CIs). The OR expresses odds in favor of success with vildagliptin or vildagliptin/metformin relative to odds in favor of success with comparator OADs. All statistical evaluations were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, North Carolina, USA. For more details please refer to the original article [26]. In this post hoc analysis only the unadjusted OR is provided.
Results
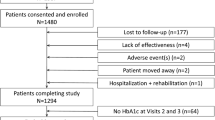
In total 754 patients were enrolled in Bulgaria, 384 in the vildagliptin cohort and 369 in the comparator cohort. One patient in the vildagliptin cohort was excluded due to inadequate source documentation or problems with quality/accuracy of data entry (Table 1). The intention-to-treat (ITT) population, used for baseline demographics and safety analyses, comprised 384 and 369 patients receiving dual therapy with newly prescribed vildagliptin or a non-vildagliptin OAD added to prior monotherapy, respectively.
The PP population was a subset of the ITT population used for the analyses of effectiveness endpoints and comprised 384 patients in the vildagliptin, and 369 in the comparator cohort.
Table 2 summarizes BL characteristics of the ITT population. Mean age of participants was 58.8 ± 9.5 years. Mean BMI was 31.9 ± 5.0 kg/m2 and mean HbA1c was comparable (vildagliptin: 8.5%; comparator: 8.2%). Mean duration of diabetes was 5.8 ± 4.8 years.
Patient populations and flow is presented in Table 1. Additional risk factors are presented in Table 3.
Table 4 reports index therapies in the ITT population by cohort. According to distribution by therapy of patients in both groups the majority of subjects in the reference group were on combination of metformin plus SU (90.8%), the second OAD added was AGI or TZD in 9% of subjects. In the vildagliptin group, majority of patients were on vildagliptin plus metformin (77.6%) combination, the rest of subjects (22.4%) were on vildagliptin plus SU combination.
PEPs and SEPs
Figure 1 shows proportions of patients achieving the PEP—HbA1c reduction >0.3%, without any tolerability issues: peripheral edema, confirmed hypoglycemia, interruption due to GI reactions, and significant weight gain >5%. The proportion of subjects successfully achieving this goal was 72.9% in the vildagliptin group versus 40.1% in the comparator group (P < 0.0001), resulting in an unadjusted OR 4.02 [95% CI 2.96–5.46].
Responder rates (per protocol population; (P < 0.001); success: treatment response without tolerability findings (HbA1c reduction—0.3%, without any tolerability issues: peripheral edema, confirmed hypoglycemia, interruption due to GI reactions, and significant weight gain >5%); failure: lack of treatment response and/or occurrence of any of the tolerability issues. Patients who could not be categorized as a success or failure (e.g., due to missing HbA1c or body weight data at 12-month endpoint) were considered non-evaluable. Non-evaluable patient data were considered failures in calculation of the odds ratio for success) (%) of Bulgarian patients achieved primary effectiveness endpoint after 12 months of treatment by groups
After 12 months of treatment, unadjusted HbA1c decreased in both cohorts (vildagliptin cohort −1.35%, comparator cohort −0.55%) (see Table 5). The drop was significantly greater with vildagliptin compared to comparator (P < 0.001) (analysis not pre-specified in protocol).
A higher proportion of patients, in the vildagliptin cohort, reached the secondary composite endpoint (HbA1c <7%, no hypoglycemic events, no weight gain) when compared to the comparator cohort (vildagliptin 32.3%, comparator 8.4%; P < 0.0001) resulting in an unadjusted odds ratio 5.2 (95% CI 3.35–8.17) (Fig. 2). The results regarding primary and secondary efficacy and tolerability endpoints are summarized in Table 6.
Responder rates (per protocol population; (P < 0.001); success: treatment response without tolerability findings (HbA1c <7%, without hypoglycemic events or weight gain); patients who could not be categorized as a success or failure (e.g., due to missing HbA1c or body weight data at 12-month endpoint) were considered non-evaluable. Non-evaluable patient data were considered failures in calculation of the odds ratio for success) (%) of Bulgarian patients achieved secondary effectiveness endpoint after 12 months of treatment by groups
Safety
The incidence of AEs was comparable between vildagliptin and comparator cohorts. Table 7 summarizes AEs and SAEs that occurred during study, listed by system organ classes (SOC). In the vildagliptin group, the incidence of AEs was 13 (3. 4%) versus 7 (1.9%) in the comparator group. The SAEs reported were 9 (2.3%) and 4 (1.1%) in the vildagliptin and comparator groups, respectively. Only one hypoglycemic event was reported in the comparator group (use of metformin and SU).
Discussion
The results of the presented sub-analysis of the EDGE study confirmed effectiveness and tolerability of vildagliptin used as a second-line OADs therapy in routine clinical settings in Bulgaria. In the Bulgarian population included in the EDGE study, the mean HbA1c drop from baseline was significantly higher with vildagliptin compared to the comparators (−1.35% in the vildagliptin cohort and −0.55% in the comparator cohort, P < 0.001). In the vildagliptin cohort, a higher proportion of patients reached the PEPs [72.9% in the vildagliptin group versus 40.1% in the comparator group (P < 0.0001)]. Current guidelines recommend that T2DM treatment should achieve target HbA1c level of <7% without increasing the risk of hypoglycemia, weight gain, and worsening cardiovascular prognosis [5, 32]. Almost four times more patients in the vildagliptin cohort reached the composite endpoint (HbA1c <7%, no hypoglycemic events, no weight gain) when compared to the comparator cohort (vildagliptin: 32.3%; comparator: 8.4%; P < 0.001). Overall, vildagliptin was well tolerated with similarly low incidences of total adverse events (3.4% versus 1.9% in the comparator group) and serious adverse events (2.3% versus 1.1% in the comparator group). The presented data regarding vildagliptin effectiveness assessed in routine clinical practice are consistent with the results from RCTs conducted until now where vildagliptin was used as an agent for oral mono- and combined therapy with other hypoglycemic agents for the treatment of T2DM [33–42]. Overall the present safety and tolerability findings in this sub-analysis of the EDGE study are in line with RCTs of vildagliptin showing no safety signals related to cardio- or cerebrovascular (CCV), pancreatitis, hepatic, immune system or skin-related disorders [33–36].
Maintaining good glycemic control without increasing the risk of CV events is essential requirement for type 2 diabetes therapy nowadays [24, 43]. The presented results regarding overall CV safety are in line with the large meta-analysis showing that vildagliptin was not associated with an increased risk of adjudicated CCV events relative to all comparators in the broad population of type 2 diabetes including patients at increased risk of CCV events [34]. A recently published meta-analysis revealed that DPP-4 inhibitors should be considered to have a neutral effect on CV outcomes [44]. Treatment with DPP-4 inhibitors compared with placebo shows no increase in risk with regards to all-cause mortality, CV mortality, acute coronary syndrome, or stroke [44]. It is yet to be seen if the results of the still ongoing large-scale cardiac outcome trials will change the current knowledge about DPP-4 inhibitors CV effects [45, 46].
The large-scale studies conducted in the recent years (ACCORD, ADVANCE and VADT) [17–19] have dramatically changed the therapeutic paradigm, going beyond the One-Glycemic-Goal-for-All concept, and showing the need to use an individualized approach in the therapy. Suboptimal glycemic control is evidenced by a number of population statistic studies on diabetes control [47], some of them conducted in Bulgaria [48]. These studies raise disputes on whether or not randomized controlled trials are a real reflection of routine clinical practice highlighting the need for real-life studies. It is in such studies where the actual effectiveness of a drug can be evaluated, which is different than its efficacy. Although used as synonyms in most cases, these two terms are different. Efficacy can be defined as the effect in ideal circumstances (RCTs). Effectiveness can be the effect in usual circumstances (real-life studies) [49]. Both RCTs and real-life studies have restrictions and should be regarded as complementing each other [50]. There is a need to know both strong and weak points of each study type. Physician’s leading decision, lack of randomization, centralized laboratory, active monitoring, etc., are among the weaknesses of the real-life studies [50].
Regardless all the potential design weaknesses, one of the biggest merits of EDGE study could be seen in the confirmation of insufficient glycemic control worldwide, despite all strong recommendations for potential benefits of the achievement of the glycemic targets in type 2 diabetes [26].
The presented sub-analysis regarding Bulgarian results from EDGE study confirmed the insufficient glycemic control too. Many factors may contribute to that suboptimal glucose control. According to the baseline characteristics of the enrolled patients, we could conclude that Bulgarian physicians decide to add second OADs at HbA1c level >8% (mean HbA1c was 8.3% ± 1.3). The delayed treatment intensification could have a harmful impact, as patients are very often left on monotherapy for too long, even if they have poor glycemic control and accumulate complications (mean disease duration was 5.8 ± 4.8 years, mean HbA1c was 8.3% ± 1.3, mean incidence of microvascular complication was 18.9% and the prevalence of underlying comorbidities as hypertension and lipid disorders was 51.1% and 19.4%, respectively).
According to the study design, patient disposition in treatment cohorts was totally based on physician discretion. In the final analysis of the EDGE trial, globally 28,442 patients in the vildagliptin group, and 15,349 patients in the reference group were included [26]. This ratio of almost 2:1 is quite different than the ratio in the Bulgarian population, which is approximately 1:1 (384 in the vildagliptin group and 369 in the comparator group). The preferred second-line OADs were SUs (90.8% of patients in the comparator cohort were on combined therapy with metformin and SU). According to the choice where to assign their patients we could conclude that Bulgarian physicians have a rather conservative attitude towards the new drugs compared to their colleagues worldwide.
The delayed and conservative approach in treatment intensification is an example of physician barriers that must be overcome before the optimal glycemic control can be obtained [51, 52]. These barriers are often referred to “clinical inertia” or “benign neglect” which describe recognition of the problem but failure to act upon it [50, 51]. Other barriers linked to poor glycemic control are treatment side effects, complex treatment regimens, needle anxiety, poor patient education, and the absence of an adequate patient care plan [53].
Timely treatment intensification combined with good treatment adherence is important for reducing the total health care system costs spent in diabetes area [54]. Non-adherence to therapy is a common problem associated with chronic diseases and one of the major barriers to optimum glycemic control in patients with T2DM, leading to poor treatment outcomes and increased utilization of health care resources [54]. Non-adherence to therapy is often related to adverse effects associated with the therapy [55]. The presented sub-analysis regarding Bulgarian results from EDGE study confirmed that vildagliptin could be used as effective and well tolerated second-line therapy. A significantly higher proportion of patients treated with vildagliptin achieved the HbA1c reduction of >0.3% without any tolerability issues: peripheral edema, confirmed hypoglycemic events, treatment interruption due to GI reactions and significant weight gain of >5%. This result is important, as it is hypoglycemia, weight gain and other AEs that lead to significant worsening of compliance and therapy adherence [55].
Furthermore, comparing the results regarding vildagliptin efficacy determined in RCTs [56] and in routine clinical practice we could conclude that the full power of treatment with vildagliptin is retained in real-life conditions in contrast to comparators with special focus on SUs. In the comparator cohort, approximately 90.8% of patients were treated with combination therapy with metformin and SUs. The HbA1c reduction seen in the comparator cohort could be evaluated as a result of the addition of SUs and was lower compared to the reduction seen in RCTs [40, 41]. A recently published analysis comparing RCTs to real-life data revealed that the decrease in HbA1c from baseline with SUs treatment is smaller in real life than in RCTs, whereas the reduction with vildagliptin is essentially the same [57]. The authors admitted that the cause of blunting of the HbA1c response to SUs in real life is unclear. They explore a hypothesis that the patient compliance with SUs therapy is reduced due to a fear of hypoglycemia and weight gain driven by defensive eating commonly associated with SUs. This could explain also the lack of aggressive dose up-titration in observational studies in which strict dosing regimen according to the study protocol is not required in comparison to RCTs [57].
Some of the study limitations could be seen in the conduct of the trial—patients were recruited both in specialized centers and by physicians working in routine care which impacted the overall number of investigators and overall results because of poor quality data and missing data which needed to be excluded from the effectiveness analyses. Safety events were likely underreported as the detection and reporting of AEs were based on the voluntary reporting scheme which is the most widely used method to identify AEs for new drugs in clinical practice. The present study is a post hoc analysis and shares all the limitations of secondary analysis such as no adjustment of the results for major potential confounders was not done (e.g., age, sex, duration, baseline HbA1c, baseline BMI).
In conclusion, this study demonstrated that in analogy to findings in the worldwide EDGE study [26], vildagliptin is both efficacious and safe when used as a second oral glucose-lowering agent in Bulgarian cohort of type 2 diabetic patients. In real-life clinical practice in Bulgaria, vildagliptin is a valid option to use in combination with metformin or any other oral glucose-lowering drug in patients with T2DM who require combination therapy. Vildagliptin also provides a greater HbA1c drop, less hypoglycemic events and a higher proportion of patients reaching target HbA1c without hypoglycemia and weight gain compared to other OADs add-on as second-line therapy.
References
Hoerger TJ, Segel JE, Gregg EW, et al. Is glycemic control improving in US adults. Diabetes Care. 2008;31:81–6.
Braga M, Casanova A, Teoh H, et al. Treatment gaps in the management of cardiovascular risk factors in patients with type 2 diabetes in Canada. Can J Cardiol. 2010;26:297–302.
IDF Diabetes Atlas, 6th ed. International Diabetes Federation: Brussels; 2013. Online version of IDF Diabetes Atlas: www.idf.org/diabetesatlas; ISBN: 2-930229-85-3. Accessed 5 Aug 2014.
De Fronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in Type 2 diabetes: A Patient-Centered Approach Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Braga MF, Casanova A, Teoh H, et al. Diabetes Registry to Improve Vascular Events [DRIVE] Investigators: Poor achievement of guidelines-recommended targets in type 2 diabetes: findings from a contemporary prospective cohort study. Int J Clin Pract. 2012;66:457–64.
Turner RC, Cull CA, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005–12.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33. Lancet. 1998;352:837–53.
Basit A, Riaz M, Fawwad A. Glimepiride: evidence-based facts, trends, and observations. Vasc Health Risk Manag. 2012;8:463–72.
Marrett E, Radican L, Davies MJ, et al. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes. 2011;4:251.
Marrett E, Stargardt T, Mavros P. Alexander CM: Patient-reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab. 2009;11:1138–44.
Hauber AB, Mohamed AF, Johnson FR, et al. Treatment preferences and medication adherence of people with type 2 diabetes using oral glucose-lowering agents. Diabet Med. 2009;26:416–24.
Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–24.
Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35:1279–84.
Fonseca VA. New developments in diabetes management: medications of the 21st Century. Clin Ther. 2014;36:477–84.
Garber AJ, Abrahamson MJ, Barzilay JI, et.al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19(2):327–36.
TheAction toControl CardiovascularRisk inDiabetes Study Group. Effects of intensive glucose lowering in type 2 Diabetes. N Engl J Med. 2008;358:2545–59.
The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 Diabetes. N Engl J Med. 2008;358:2560–72.
Duckworth W, Abraira C, Moritz T, VADT Investigators, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol. 2009;62:499–505.
Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials. 2009;10:37.
Ware JH, Hamel MB. Pragmatic trials–guides to better patient care? N Engl J Med. 2011;364:1685–7.
McIntosh B, Cameron C, Singh SR, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5:e35–48.
Ray KK, Seshasai SRK, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72.
Scheen AJ. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10:73–84.
Mathieu C, Barnett AH, Brath H, et al. Effectiveness and tolerability of second-line therapy with vildagliptin vs. other oral agents in type 2 diabetes: a real-life worldwide observational study (EDGE). Int J Clin Pract. 2013;67(10):947–56.
American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–66.
World Health Organization. Gaining health: the european strategy for the prevention and control of noncommunicable diseases. 2006. http://www.euro.who.int/__data/assets/pdf_file/0008/76526/E89306.pdf. Accessed 05 August 2014.
Borisova AM, Shinkov A, Vlahov J et al. Screening of prevalence of endocrinology diseases in Bulgarian population (≥20–80 age), preliminary results. In: 15th National Symposium on Endocrinology. 2012.
National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight adults and obesity in adults. http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.htm (1998). Accessed on 05 Aug 2014.
Vildagliptin SPC. EMA available on http://ec.europa.eu/health/documents/community-register/2013/20130731126393/anx_126393_en.pdf. Accessed 05 Aug 2014.
Standards of Medical Care in Diabetes—2012. American Diabetes Association. Diabetes Care. 2012; 35(Suppl 1):S11–63.
Ligueros-Saylan M, Foley JE, Schweizer A, et al. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes Metab. 2010;12(6):495–509.
Schweizer A, Dejager S, Foley JE, et al. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabete Obes Metab. 2010;12(6):485–94.
Schweizer A, Dejager S, Foley JE, et al. Assessing the general safety and tolerability of vildagliptin: value of pooled analyses from a large safety database versus evaluation of individual studies. Vasc Health Risk Manag. 2011;7:49–57.
He YL, et al. Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin Pharmacokinet. 2012;51(3):147–62.
Bader G, Geransar P, Schweizer A. Vildagliptin more effectively achieves a composite enpoint of HbA1c <7% without hypoglycaemia and weight gain compared with glimepiride after 2 years of treatment. Diabetes Res Clin Pract. 2012.
Bolli G, Dotta F, Colin L, et al. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab. 2009;11:589–95.
Bolli G, Dotta F, Rochotte E, et al. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10:82-90.
Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–66.
Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with Type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med. 2010;27:318–26.
Garber AJ, Schweizer A, Baron MA, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–74.
US Department of Health and Human Services: Food and Drug Administration. Diabetes Mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008; 1–5. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed 05 Aug 2014.
Wu S, Hopper I, Skiba M, et al. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther. 2014;32:147–58.
Bethel M, Green J, Califf R, Holman R. Rationale and design of the trial evaluating cardiovascular outcomes with Sitagliptin (TECOS) (abstract). Diabetes Care. 2009;58(Suppl. 1):2152.
Rosenstock J, Marx N, Kahn S, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diabetes Vasc Dis Res. 2013;10:289–301.
Berkowitz SA, Meigs JB, Wexler DJ. Age at type 2 diabetes onset and glycaemic control: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2010. Diabetologia. 2013;56(12):2593–600.
Hristov V, Kamenov Z, Georgiev B. Quality of Glycemic Control in Bulgaria awareness and reality (from theory to practice). Endocrinology. 2006;4(223–33):38.
Marley J. Efficacy, effectiveness, efficiency. Aust Prescr. 2000;23(6):114–7.
Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. European Medicines Agency. 2012. www.ema.europa.eu. Accessed 05 August 2014.
Allen JD, Curtiss FR, Fairman KA. Non adherence, clinical inertia, or therapeutic inertia? J Managed Care Pharm. 2009;15(8):690–5.
Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–34.
Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–43.
Berger J. Economic and clinical impact of innovative pharmacy benefit designs in the management of diabetes pharmacotherapy. Am J Manag Care. 2007;13:S55–8.
Cryer PE, Childs BP. Negotiating the barrier of hypoglycemia in diabetes. Diabetes Spectr. 2002;15:20–7.
Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30(4):890–5 (Epub 2007 Feb 2).
Ahrén B, Mathieu C, Bader G. Efficacy of vildagliptin versus sulfonylureas as add-on therapy to metformin: comparison of results from randomised controlled and observational studies. Diabetologia. 2014;57(7):1304–7. doi:10.1007/s00125-014-3222-z (Epub 2014 Mar).
MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 14.0. Chantilly, Virginia. 2011; MSSO-DI-6003-14.0.0.
Acknowledgments
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Sponsorship and article processing charges for this study were funded by Novartis Pharma AG. Sponsor and Steering Committee had equal roles in determining study design, protocol finalization and data interpretation. The sponsor was fully responsible for protocol writing, data collection, and analysis. All authors had final responsibility for the data, content, and decision to submit for publication. The author meets the ICMJE criteria for authorship for this manuscript, takes responsibility for the integrity of the work as a whole, and has given final approval for the version to be published. Novartis gratefully acknowledges all the Bulgarian clinical centers participating in the EDGE study in the face of their principal investigators: Sabina Zaharieva; Natalya Temelkova; Zdravko Kamenov; Galina Dakovska; Ivona Daskalova; Malina Petkova; Ludmila Lubenova; Mihail Protich; Galina Lazarova; Emilia Kovandgieva; Miglena Kostova; Kiril Hristozov; Ludmila Staneva; Penka Velkova; Nikolay Stoyanov; Stefka Vladeva; Hristo Georgiev; Albena Kichukova; Mariya Pavlova; Donka Mutafchieva; Boyan Manolov; Tsvetodara Kuneva; Mariya Lucheva; Margarita Temelkova.
Conflict of interest
Zdravko Kamenov has participated as an investigator in studies conducted by Novo Nordisk; Novartis Pharma; Merck Serono; MSD; Sanofi Aventis. Z. Kamenov had speaker engagements for Novartis Pharma; Boehringer Ingelheim; MSD; Astra Zeneka; Novo Nordisk; Ely Lilly; Sanofi Aventis; Merck Serono; Berlin Chemie, Actavis; Abbott.
Compliance with ethics guidelines
The present sub-analysis does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kamenov, Z. Effectiveness and Tolerability of Second-Line Therapy with Vildagliptin Versus Other Oral Agents in Type 2 Diabetes (EDGE): Post Hoc Sub-Analysis of Bulgarian Data. Diabetes Ther 5, 483–498 (2014). https://doi.org/10.1007/s13300-014-0083-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-014-0083-x