Abstract
Introduction
In the Liraglutide Effect and Action in Diabetes (LEAD) randomized clinical trials (RCTs) assessing liraglutide in type 2 diabetes mellitus (T2DM), glycated hemoglobin (A1c) was reduced by 7–16 mmol/mol and weight by up to 3.4 kg. As real-life efficacy data on liraglutide is limited, the authors assessed clinical effects in a real-life cohort.
Methods
In this retrospective analysis from the Israeli Health Maintenance Organization Maccabi, of patients with T2DM, treated with liraglutide ≥6 months during 2011–2012, evaluations were performed at baseline and 6 months.
Results
Insulin-naïve patients (n = 1,101) treated with liraglutide with at least one A1c or weight measurement were identified. In 933 patients with an additional A1c value after 6 months, A1c decreased by 9 mmol/mol (p < 0.0001, 95% CI 7–11) from 72 mmol/mol. In patients receiving >2 oral antidiabetic drugs (OADs) prior to liraglutide treatment (80.7% patients), A1c decreased by 7 mmol/mol, and in those receiving ≤2 OADs, by 12 mmol/mol. In 453 patients with baseline data available, weight decreased by 2.55 kg (p < 0.0001); 173 patients (38.18%) achieved ≥1% A1c reduction. Furthermore, 91 patients (20.1%) achieved National Institute for Health and Care Excellence (NICE) criteria (decreased A1c ≥1%; weight ≥3%). Weight reduction was marginally correlated with A1c reduction.
Conclusions
Evidence from real-life use of liraglutide demonstrated clinical effects similar to those demonstrated in RCTs.
Similar content being viewed by others
Introduction
Better understanding of the pathophysiology of type 2 diabetes mellitus (T2DM) and the central role of the incretins in glucose metabolism led to the development of glucagon-like peptide 1 (GLP-1) receptor agonists as therapeutic agents [1]. In randomized controlled clinical trials, the use of GLP-1 agonists in patients with T2DM caused a substantial decrease in blood glucose and glycated hemoglobin (A1c) measures, combined with weight loss and a low incidence of hypoglycemia [2, 3]. These merits likely contributed to worldwide acceptance of GLP-1 agonists by physicians and patients alike, despite the need for delivery by injection. In current treatment guidelines, therapy for T2DM includes GLP-1 agonists as an equal or superior treatment option compared with classic oral agents. The American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) position statement includes GLP-1 agonists in one of the five combinations for dual therapy and in four combinations for triple therapy [4]. GLP-1 agonists are the top prioritized class after metformin for monotherapy, dual therapy, and triple therapy in the American Association of Clinical Endocrinologists (AACE) algorithm [5].
Liraglutide is one of the leading GLP-1 agonist therapeutic options [6]. It is a GLP-1 analog that shares 97% sequence homology to native GLP-1. The addition of a C16 fatty acid side chain enables once-daily dosing of liraglutide by prolonging its duration of action to over 24 h. The safety and efficacy of liraglutide have been well detailed in the phase 3 trials, the Liraglutide Effect and Action in Diabetes (LEAD) program [7–12]. Data from the LEAD trials have demonstrated that liraglutide effectively improves glycemic control in individuals with T2DM, when used as monotherapy, or in combination, with one or more selected oral antidiabetic drugs (OADs). Across the trials, mean body weight decreased with liraglutide treatment. Reductions in systolic blood pressure (SBP) and improvements in lipid profiles were also observed across the trials. However, these well-designed randomized clinical trials (RCTs) conducted under strict inclusion and exclusion criteria provide limited information about the efficacy of liraglutide in selected populations. Moreover, cost-effectiveness issues and limited budget have led payers to impose restrictions on the use of liraglutide that were not part of the patient selection in the RCTs and that may influence the outcome in treated patients. Retrospective insurance-based databases and electronic medical records analyses can provide information and guidance beyond that provided in the clinical trials for both payers and prescribers. Recently, reports on real-life effects of liraglutide have been published, but these are based on a small number of patients in a limited number of clinics [13, 14]. In this study, the authors analyzed the effects of liraglutide use in patients with T2DM in a leading Israeli Health Maintenance Organization (HMO) using their large, comprehensive database in an attempt to confirm effectiveness of liraglutide in a real-world setting when prescribed under payers’ restrictions.
Subjects
Setting
This retrospective health claim and electronic medical records analysis was conducted in Maccabi Healthcare Services (MHS), the second-largest HMO in Israel, serving 25% of the total population countrywide (about 2 million members). Since 1997, information on all members’ interactions (i.e., diagnoses, visits to primary and secondary care physicians, visits to outpatient clinics, hospitalizations, laboratory tests, and purchased and dispensed medications) have been downloaded daily to a central computerized database. In addition, MHS has developed and validated computerized registries of its patients suffering from major chronic diseases such as ischemic heart disease, oncological diseases, and diabetes [15, 16].
Patients
The inclusion criteria to the diabetes registry are all patients who have one or more of the following: A1c ≥55.7 mmol/mol, blood glucose ≥11.1 mmol/L, a preceding diagnosis of diabetes according to any relevant International Classification of Diseases, 9th revision (ICD-9) codes [17] and A1c ≥48 mmol/mol or glucose >6.9 mmol/L, or have purchased hypoglycemic medication twice within the last 2 months. Similar to previously described diabetes registries, definitions of type 1 diabetes (T1D) and T2DM are based on accessible data in the electronic files (e.g., age of the patient and treatment) and not on diagnosis as both types have identical ICD-9 code. Patients are identified by an automated database search and therefore the registry is not dependent on physicians actively reporting on the patient to the registry. The diabetes registry holds information for >90,000 patients with diabetes. According to the 1994 Israel National Health Act, MHS may not deny coverage to applicants on any grounds, including age or state of health. Thus, all sectors of the Israeli population are represented in MHS, except for young adults aged 18–21 years, due to a high percentage of them being enlisted in the Israeli Defence Forces (IDF), and therefore receiving medical care there.
The study was approved by Maccabi’s ethics committee and was performed in accordance with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Methods
Treatment was assessed by evaluating drug purchases obtained from Maccabi’s pharmacies. As drugs are purchased 3 months in advance, the authors could not assess the dose that patients were actually taking. During this period, reimbursement rules for liraglutide prescription were body mass index (BMI) >30 kg/m2 and A1c >63.9 mmol/mol after use of at least two OADs. All data were obtained retrospectively from patient medical records, and reflect the routine practice in the HMO during this time period. To be included, patients had to have T2DM and be treated with liraglutide for 6 months or more. Prescription of liraglutide was performed as ‘add-on’ therapy for most patients. A minority of patients were switched from dipeptidyl peptidase-4 (DPP-4) inhibitors or insulin. Patients with T1D were excluded, as were patients with cancer, end stage liver disease, end stage renal failure (non-diabetes related), female patients with gestational diabetes, and patients treated concomitantly with insulin or DPP-4 inhibitors.
Data extracted for the study included sociodemographic details, diabetes duration, diabetes treatment since 2009, weight, height, SBP, comorbidities and laboratory results of A1c, and lipid profile. The authors also had information on whether and when each patient was included in Maccabi’s cardiovascular registry and chronic kidney disease registry. Evaluations for all variables were performed at baseline (within 180 days prior to liraglutide first prescription date) and at 6 months ± 90 days. The authors also had information on whether patients were included in the cardiovascular registry. The cardiovascular registry includes all patients who have been diagnosed twice or more by hospital or outpatient cardiologists, primary physicians, or pediatricians with at least one of the following clinical diagnoses, classified according to the ICD-9 codes: ischemic heart disease; myocardial infarction; congestive heart failure; peripheral vascular disease; cerebrovascular disease; transient ischemic attack; atrial fibrillation; prior coronary artery bypass grafting; or percutaneous coronary intervention. The chronic kidney disease registry included all who had a glomerular filtration rate (GFR) <60, or a GFR ≥60 and two urine tests at least 3 months apart with proteinuria (protein/creatinine ratio greater than 45 mg/mmol, which is equivalent to albumin/creatinine ratio greater than approximately 300 mg/g).
Evaluations for all variables were performed at baseline (within 180 days prior to liraglutide first prescription date) and at 6 months ± 90 days. When restricting the analysis to 90 days prior to liraglutide prescription, results were similar but the sample size was smaller.
Statistical Methods
Descriptive statistics of patient data was performed and expressed as means and standard deviations (SD) for continuous variables and as number and percentage for dichotomous variables. Results of continuous variables were compared using paired Student’s t test. Statistical significance was set at p < 0.05.
Forest plots were calculated for the reduction in A1c and body weight in different subgroups of patients, and in relation to possible determinants of glycemic efficacy and weight reduction after starting liraglutide treatment. A Student’s t test was used when there were two conditions, and an analysis of variance (ANOVA) was used when there were more conditions. p was considered significant if <0.05 and confidence intervals were calculated. Correlation between variables was assessed using Pearson coefficients.
To assess determinants of changes in A1c and weight, a univariate analysis was performed. Dependent variables were changes in A1c and weight. Independent variables were age, gender, diabetes duration, number of previous OADs, baseline A1c, baseline weight, cardiovascular comorbidity, chronic kidney disease, baseline low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides and SBP. To enter the multivariate regression model p was set at <0.2. Significance was set at p < 0.05.
Results
One thousand one hundred and one patients fulfilled the inclusion and exclusion criteria and had at least one weight or A1c determination within the specified time frames. For 933 patients, the authors had two measurements of A1c in the appropriate time frame at baseline and 6 months after starting liraglutide treatment. The characteristics of these patients are presented in Table 1. Mean age was 59.71 (SD 8.99) years, 53.5% were male, and mean duration of diabetes was 9.83 (SD 3.29) years. Baseline A1c was 72 mmol/mol (SD 14), and baseline weight and BMI, available in 453 patients, were 98.03 kg (SD 17.57) and 34.65 kg/m2 (SD 5.00), respectively. Cardiovascular comorbidity was present in 28.51% of patients, and 38.69% had chronic kidney disease. Baseline LDL was 2.27 mmol/L (SD 0.68) and baseline triglycerides were 2.40 mmol/L (SD 1.44). Baseline SBP was 135.40 mmHg (SD 16.21).
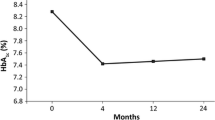
Liraglutide treatment had a significant effect on patients’ A1c (p < 0.0001). After 6 months of treatment, A1c had decreased by 9 mmol/mol (SD 13) (95% CI 7–11) (Table 2). In addition, weight decreased by 2.55 kg (SD 4. 26) (95% CI 2.15–2.94), and BMI by 0.90 kg/m2 (SD 1.49), (95% CI 0.76–1.03). Liraglutide also significantly reduced SBP by 3.50 mmHg (SD 17.13) (95% CI 2.22–4.78), while LDL decreased by 0.09 mmol/L (SD 0.69) (CI 0.03–0.14) and triglycerides by 0.1 mmol/L (SD 1.30), (96% CI 0.01–0.19). HDL levels remained stable.
Seventy-eight percent of patients decreased their A1c in response to liraglutide treatment. Altogether, 55% of patients had a decrease in A1c of at least 11 mmol/mol; of these, 15% had a decrease of at least 22 mmol/mol. Fifty-six percent of patients lost 2 kg or more, with 43% losing 3 kg or more (Fig. 1). Ninety-one patients (20.1%) achieved the National Institute for Health and Care Excellence (NICE) criteria (decrease of A1c ≥11 mmol/mol and weight reduction ≥3%). Of note, the correlation between A1c reduction and weight reduction was significant, but of a small magnitude (Pearson correlation 0.1156, p = 0.0139) (Fig. 2).
As can be seen in Table 1, 80.7% of patients received more than two OADs prior to liraglutide treatment.
The 75.7% of patients who received a DPP-4 inhibitor were further evaluated. Table 2 presents the effect of liraglutide treatment according to the number of OADs received prior to therapy and the association with prior DPP-4 inhibitor treatment. Effect of liraglutide on A1c reduction as well as weight reduction and reduction in BMI remained significant in patients who received more than two OADs prior to starting liraglutide treatment, though the magnitude of A1c reduction was somewhat smaller compared with patients who received two OADs or fewer. Furthermore, even patients previously treated with a DPP-4 inhibitor demonstrated significant A1c, weight and BMI reduction on liraglutide treatment.
Subgroup analyses showed no significant differences in liraglutide’s effect by gender, weight, age or diabetes duration. In contrast, there was a significant relation between baseline A1c and A1c reduction (<0.0001), as higher A1c levels were significantly related to a higher reduction in A1c (Fig. 3a). There were no significant correlations between gender, age, BMI, baseline A1c and diabetes duration and weight reduction (Fig. 3b).
Forest plots reporting reduction in A1c (a) and weight (b) after 6 months of liraglutide treatment in different subgroups of patients. p values are shown for t test when there were two conditions or for ANOVA when there were three tertiles. A1c glycated hemoglobin, BMI body mass index, DPP-4 dipeptidyl peptidase-4
The authors further tried to assess the variables determining the degree of A1c reduction. In the univariate analysis (Table 3) there was a strong positive correlation between A1c reduction and baseline A1c, and the number of prior OADs. When entering these variables into a multivariate linear regression model, variables that remained significantly correlated to A1c reduction were baseline A1c, cardiovascular comorbidity and the number of prior OADs (Table 4). The authors also calculated an additional multivariate model for A1c reduction where, in addition to the above-mentioned variables, the authors also entered baseline LDL, HDL, triglycerides and baseline SBP. These variables did not contribute significantly to the model, but limited significantly the number of patients assessed in the model.
Variables that significantly affected weight reduction in the univariate analyses were baseline weight, and the number of OADs the patient took prior to liraglutide treatment (Table 5). The authors also calculated an additional multivariate linear regression model with a dependent variable of weight reduction. None of the multivariate models created for weight reduction were significant.
Discussion
In this real-world study, liraglutide was shown to be an effective treatment for diabetes, leading to a 9 mmol/mol reduction in A1c accompanied by 2.55 kg reduction in weight (Table 2). In 55% of these patients, the reduction was at least 11 mmol/mol and a weight reduction of >3 kg was observed in 43% of patients (Fig. 1). Twenty percent of patients with full laboratory and weight data achieved the NICE criteria for effectiveness [18]. Information on liraglutide efficacy is mainly based on a series of randomized, controlled clinical registration trials [7–12] (the LEAD trials) conducted over time periods ranging in duration from 26 to 52 weeks. This trial program was comprehensive and included 5,796 patients and investigating a number of active comparators covering a wide range of therapeutic options in the spectrum of T2DM [19]. Liraglutide, administered as monotherapy or in combination with other OADs, was compared with insulin glargine, exenatide, glimepiride and various combinations of glimepiride, metformin and rosiglitazone. Diverse T2DM populations were studied across the trials, ranging from those who were treatment-naïve to those who had been failing to achieve glycemic targets using multiple OADs.
In this program, liraglutide was shown to reduce A1c levels by 9–18 mmol/mol from baseline, and weight by up to 3.4 kg [20]. Patients had disease duration of 7.7 years, and a baseline A1c of 68 mmol/mol (66–69 mmol/mol). Thus, the results seen in the current cohort show a reduction of A1c that is somewhat lower than those recorded in the RCTs. A plausible explanation for this difference is that patients in our cohort were older (59.7 years as opposed to 56 years), had longer disease duration (9.83 years as opposed to 7.7 years), and had greater weight (98 kg as opposed to 90 kg) than patients studied in the RCTs. This probably reflects the fact that reimbursement was limited to those patients with an A1c greater than 63.9 mmol/mol and a BMI greater than 30 kg/m2. Liraglutide effect on A1c in this cohort falls in the lower range of A1c and weight observed in the RCTs probably because this population was not only older with longer disease duration but also received more than two OADs, including DPP-4 inhibitors. The importance of early initiation of liraglutide is underscored by the effect liraglutide had in patients who received prior therapy with ≤2 OADs (12 mmol/mol and −3 kg). The higher effect in this group is in line with a meta-analysis of the LEAD studies where patients on ≤1 OAD had a much higher reduction in A1c when compared to patients receiving two OADs (15 vs. 9 mmol/mol on liraglutide 1.2 mg and 17 mmol/mol vs. 13 mol/mol on liraglutide 1.8 mg treatment [21]). Moreover, those treated with liraglutide after diet or monotherapy had a better chance to achieve a composite target of A1c <53 mmol/mol with no weight gain when treated with liraglutide, compared with those who received the GLP-1 agonist after treatment with various combination therapies [22]. It should be emphasized that 75.7% of patients in this cohort had previously taken a DPP-4 inhibitor and were subsequently switched to liraglutide because of deteriorating A1c or a failure to reach therapeutic goals. Despite this, 78% of these patients had a further A1c reduction and in 55% the reduction was at least 11 mmol/mol.
This study is the largest real-life study published so far on use of liraglutide and is based on a large database of a nationwide HMO that reflects the Israeli population. Other smaller studies published to date are in line with the findings of this study. In a study from 16 clinics in Wales, 1,114 patients using GLP-1-based therapies were followed for a median of 48 weeks. Of the 256 who received liraglutide 1.2 mg, NICE treatment continuation criteria (≥11 mmol/mol HbA1c reduction, ≥3% weight loss) were met by 32% [13]. A further real-world study followed 166 patients from three clinics [14]. Patients had a baseline A1c of 72 mmol/mol and BMI of 36.34 kg/m2. Mean follow-up was 9.4 (SD 4.2) months (range 4–16). Patients lost on average 16 mmol/mol A1c and 4.0 kg body weight. Significant independent determinants of A1c drop were baseline A1c (r = 0.673; p < 0.001) and previous insulin therapy (r = -0.251; p < 0.001). The only independent determinant of weight loss was baseline BMI (r = 0.429; p < 0.001). In this study, it has been found that baseline A1c and number of previous OADs had a significant explanatory role. This is in line with the overall impression that early use of GLP-1 agonists could lead to greater benefits. In the Italian study, the drop in A1c was unrelated to baseline BMI or weight loss, while in this current study there was a small but significant correlation between the degree of weight lost and reduction in A1c. This suggests that most of the drop in A1c is independent of weight change. Rather it seems that when weight loss occurs it may further reduce glucose levels.
A recent review that assessed available evidence from clinical trials regarding the efficacy and safety of GLP-1 agonists in the first- or second-line management of T2DM suggests that the early (i.e., second-line, or, in some cases first-line) use of liraglutide and exenatide is justified on grounds of efficacy and safety [23].
This study has several limitations. This was a non-interventional observational study, in accordance with the definition applied by the European Medicines Agency (Directive 2001/20/EC) [24]. Study-specific patient visits, tests and monitoring were not imposed, and only data originating from routine clinical practice were collected. As data were obtained from observational registries, clinical events may not have been captured in full and patient follow-up was not as tight as would be expected in an RCT. In addition, the time relationship between liraglutide administration and the laboratory data was more flexible compared with an RCT. Missing laboratory data and other measurements such as weight and blood pressure were not always available in the specified time frame. Indeed the time frame chosen for a clinical trial would have been shorter and closer to the start and end points. The heterogeneity of baseline characteristics means that between group comparisons, such as those regarding A1c change, should be interpreted with caution.
On the other hand, this study has many strengths. The large size of this study (made feasible by undertaking this research in a non-interventional manner) and the limited exclusion criteria increase the robustness of the findings and potentially improve generalizability of liraglutide effect to the broader population. The recognized quality of this well-established electronic medical record, the automatic data capture and use of one central laboratory increase the confidence in this database.
Conclusion
Evidence from real-life use of liraglutide demonstrated significant reductions in A1c, weight, SBP and improved lipid profile, supporting the clinical effect of liraglutide demonstrated in RCTs. In many ways the effectiveness of liraglutide in this real-world study was greater than may have been anticipated in such a cohort. Therefore, this study suggests the adoption of a liberal prescription policy for liraglutide, particularly for the difficult-to-treat patients.
References
Knudsen LB. Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem. 2004;47:4128–34.
Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, 1860 LIRA-DPP-4 Study Group, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56.
Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–60.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–36.
Unger J. Liraglutide: can it make a difference in the treatment of type 2 diabetes? Int J Clin Pract. 2010;64(Suppl 167):1–3.
Marre M, Shaw J, Brandle M, Wan Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268–78.
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin in type 2 diabetes mellitus (LEAD-2 Met). Diabetes Care. 2009;32:84–90.
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 mono): randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81.
Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human GLP-1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met + TZD). Diabetes Care. 2009;32:1224–30.
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. A study of two glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: liraglutide once daily compared with exenatide twice daily in a randomised, 26-week, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Evans M, McEwan P, O’Shea R, George L. A retrospective, case-note survey of type 2 diabetes patients prescribed incretin-based therapies in clinical practice. Diabetes Ther. 2013;4:27–40.
Fadini GP, Simioni N, Frison V, Dal Pos M, Bettio M, Rocchini P, et al. Independent glucose and weight-reducing effects of Liraglutide in a real-world population of type 2 diabetic outpatients. Acta Diabetol. 2013;50:943–9.
Heymann A, Chodick G, Halkin H, Karasik A, Shalev V, Shemer J, et al. The implementation of managed care for diabetes using medical informatics in a large Preferred Provider Organization. Diabetes Res Clin Pract. 2006;71:290–8.
Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann A. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol. 2011;152:345–9.
World Health Organization. The international classification of diseases, 9th revision, clinical modification (ICD-9-CM), sixth edition. http://www.who.int/classifications/icd/en/. Accessed 10 March 2014.
NICE Technology Appraisal Guidance 203. Liraglutide for the treatment of type 2 diabetes mellitus. Oct 2010. http://www.nice.org.uk/guidance/TA203. Accessed 27 October 2013.
Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract. 2012;97:27–42.
Niswender K, Pi-Sunyer X, Buse J, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15:42–54.
Garber AJ, Matthews D, Zinman B, Thompson AB, Falahati A, Guerci B. The effect of disease stage, indicated by number of previous oral antidiabetic agents, on the response to liraglutide in type 2 diabetes. Diabetes. 2011;60 (Suppl 1):A265 (Abstract 967P).
Ratner R, Brett J, Khurtoryansky N, Aroda VR. Identifying predictors of response to liraglutide in type 2 diabetes using recursive partitioning analysis. Diabetologia. 2012;55(Suppl 1):S332 (Abstract 806-P).
Ross SA, Ballantine J. Early use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in Type 2 diabetes. Curr Med Res Opin. 2013;29:1617–26.
European Medicines Agency. Directive 2001/20/EC. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:121:0034:0044:en:PDF. Accessed 27 October 2013.
Acknowledgments
Sponsorship and article processing charges for this study was funded by Novo Nordisk. The authors wish to thank Jenna Steere of Watermeadow Medical (Oxford UK) for providing technical writing assistance. This was funded by Novo Nordisk. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
A. Heymann has been paid for consultancy services by Novo Nordisk. Y. Maor has been paid for consultancy services by Novo Nordisk. L. Todorova is a Novo Nordisk employee. P. Schertz-Sternberg is a Novo Nordisk employee. A. Karasik has been paid for consultancy services by, and is part of Speakers office for Novo Nordisk, Merck, Boehringer Ingelheim, Lilly, Astra Zeneca and Novartis. I. Goldstein has no conflict of interest to declare.
Compliance with ethics
The study was approved by Maccabi’s ethics committee and was performed in accordance with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Heymann, A., Maor, Y., Goldstein, I. et al. Efficacy of Liraglutide in a Real-Life Cohort. Diabetes Ther 5, 193–206 (2014). https://doi.org/10.1007/s13300-014-0062-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-014-0062-2