Abstract
A generalized skin eruption with strong itching was induced by sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, in a patient almost 6 months after initiation of the drug. Physical examination revealed a spread of skin rash from chest to back, and abdomen and thigh. Discontinuation of the drug eliminated the skin rash immediately. The emergence of new rash ended, and the rash itself withered after 1 week. The spread of the rash gradually shrank and the skin lesions subsided, leaving pigmentation 1 month later. Two months after cessation of sitagliptin, the skin eruption had subsided and oral steroid medication was stopped, but some small eczematous eruptions continued to appear intermittently. Although a drug-induced lymphocyte stimulation test was negative for sitagliptin, nonspecific radioimmunosorbent test for immunoglobulin E was increased to 532 IU/mL, with a percentage of eosinophil of 7.4%. Sitagliptin has a phenyl ring, carbonyl group, and an absorption spectrum showing three absorption peaks (199.9, 265.0, 400.1 nm), and its photosensitive mechanism could have been responsible for the itchy edematous plaque. In the present case, the initial generalized skin eruption may have been induced by an allergic reaction to sitagliptin. Close attention should be paid to patients receiving this drug with a history of urticaria, and to the development of photosensitivity.
Similar content being viewed by others
Introduction
The increasing number of patients with type 2 diabetes mellitus is a prominent health problem worldwide. Incretin-based therapy is one of the promising new treatments for type 2 diabetes mellitus, and has recently become a first-line drug. Although side effects do not often appear with this class of drug, several adverse events have been reported so far [1, 2]. Here, the authors report a case of generalized drug-induced skin eruption with strong itching nearly 6 months after initiation of sitagliptin.
Case Report
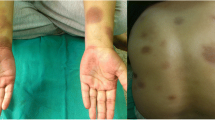
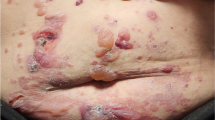
A 66-year-old male with untreated type 2 diabetes mellitus [3] was admitted to the authors’ hospital following the advice of the patient’s daughter. The patient’s hemoglobin A1c (HbA1c) level had been 7.4% in a general health check-up 3 years before. One year prior to admission, the patient’s fasting glucose had risen to 126 mg/dL and his HbA1c level rose up to 8.6%. The patient had a history of urticaria several years earlier. A diet and exercise regimen was introduced, and sitagliptin phosphate 50 mg and metformin 500 mg were started. Two months later, the patient’s HbA1c level had improved to 7.0% and the patient continued on the medication, and diet and exercise therapy. Six months later, a rash with a locus on the upper limb began to appear. The patient applied antihistamine ointment on the skin rash, which continued to spread gradually from chest to back, and abdomen to thigh (Figs. 1a, 2a, 3a, b). In some areas of the back and chest, lichenification also appeared. The itching associated with the rash also worsened, interfering with sleep during the night. The patient consulted a dermatologist, and oral and ointment steroids were started. However, the rash was unchanged and pruritus gradually increased. Since there is a possibility of skin malignancy in eczematous skin rashes lasting for a long period of time, a skin biopsy was scheduled. Four months after the eruption first appeared, and just before the skin biopsy, the authors stopped the dipeptidyl peptidase-4 (DPP-4), sitagliptin, to rule out the possibility of a drug reaction, although metformin was continued. Itching caused by the rash was significantly relieved immediately after discontinuation of the drug. The emergence of new rash ended, and the rash itself withered after 1 week. The spread of the rash gradually shrank and the skin lesions subsided, leaving pigmentation 1 month later (Figs. 1b, 2b). Although discontinuation of sitagliptin was significantly effective for the skin rash, a drug-induced lymphocyte stimulation test was negative for sitagliptin. Nonspecific radioimmunosorbent test for immunoglobulin E was increased to 532 IU/mL, and the percentage of eosinophil was 7.4%. Two months after cessation of sitagliptin, the skin eruption had subsided (Figs. 1c, 2c) and oral steroid medication was stopped, but some small eczematous eruptions continued to appear intermittently.
Discussion
Sitagliptin was the first DPP-4 inhibitor approved by the US Food and Drug Administration (FDA) in October 2006, for the treatment of type 2 diabetes mellitus. Of the spontaneous adverse event reports of hypersensitivity reactions (such as anaphylaxis, angioedema, and serious skin reactions), most have occurred within the first 3 months after initiation of the treatment, with many following the first dose [4]. According to the Uppsala Monitoring Centre (a World Health Organization collaborating center) causality assessment system [5], the category for the present case is “certain,” although the appearance of the adverse event occurred about 6 months after sitagliptin initiation. In the literature, skin rash induced by sitagliptin has so far been reported in only two cases, one of persistent edematous-plaque photosensitivity [6] and another of bullous pemphigoid [7]; the former a cutaneous eruption induced by a photosensitive reaction to sitagliptin that appeared about 2 weeks after starting sitagliptin but continued for almost 2 years after cessation. In the present case, the eruption appeared almost 6 months after initiation of the drug and gradually subsided after the cessation, except for small itchy erythematous eruptions that continue to appear intermittently around the sun-exposed upper chest area. Because sitagliptin, like all known photosensitizers, has a phenyl ring, carbonyl group, and an absorption spectrum showing three absorption peaks (199.9, 265.0, and 400.1 nm), its photosensitive mechanism could well be responsible for the itchy edematous plaque. While both the 199.0 and 265.0 nm wavelengths are within the UV-C spectrum, the 400.1 nm absorbance peak indicates that sitagliptin also absorbs UV-A-visible light. Thus, sitagliptin could cause persistent photosensitive eruption after cessation of the drug even with protection from UV light by hapten formation with subcutaneous protein.
In the present case, the distribution of skin rash differs from that of typical photosensitivity, of which there is no proof of involvement. Although a skin biopsy was not done in this case, varied sizes of bullae were not observed throughout the course of the rash, which contraindicates bullous pemphigoid. In addition, the patient’s history of urticaria before using sitagliptin is relevant. Although metformin was used throughout the affected period, the skin eruption occurred only during the use of sitagliptin. The reports of drug-induced rash for metformin are very few despite its long history of use. In the literature, only psoriasiform [8] and leukocytoclastic vasculitis [9] have been reported to be induced by metformin. More observation is required over a period of time to clarify use of this class of drug and also for the outcome of this case.
Conclusion
In the present case, the initial generalized skin eruption may have been induced by an allergic reaction to sitagliptin. Close attention should be paid to patients receiving this drug with a history of urticaria, and to the development of photosensitivity.
References
Williams-Herman D, Round E, Swern AS, et al. Safety and tolerability of sitagliptin in patients with type 2 diabetes: a pooled analysis. BMC Endocr Disord. 2008;8:14.
Nauck MA, Vilsøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care. 2009;32:S223–31.
Seino Y, Nanjo K, Tajima N, et al. The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–28.
Deasi S, Brinker A, Swann J, Iyasu S. Sitagliptin-associated drug allergy: review of spontaneous adverse event reports. Arch Intern Med. 2010;170:1169–71.
Uppsala Monitoring Centre. The use of the WHO-UMC system for standardized case causality assessment. Available at: http://who-umc.org/Graphics/24734.pdf. Accessed 28 Aug 2012.
Stricklin SM, Stoecker WV, Rader RK, Hood AF, Litt JZ, Schuman TP. Persistent edematous-plaque photosensitivity observed with sitagliptin phosphate (Januvia®). Dermatol Online J. 2012;18:9.
Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249–53.
Koca R, Altinyazar HC, Yenidünya S, Tekin NS. Psoriasiform drug eruption associated with metformin hydrochloride: a case report. Dermatol Online J. 2003;9:11.
Ben Salem C, Hmouda H, Slim R, Denguezli M, Belajouza C, Bouraoui K. Rare case of metformin-induced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685–7.
Acknowledgments
Dr. Kurose is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
The authors declare no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nakatani, K., Kurose, T., Hyo, T. et al. Drug-Induced Generalized Skin Eruption in a Diabetes Mellitus Patient Receiving a Dipeptidyl Peptidase-4 Inhibitor Plus Metformin. Diabetes Ther 3, 14 (2012). https://doi.org/10.1007/s13300-012-0014-7
Received:
Published:
DOI: https://doi.org/10.1007/s13300-012-0014-7