Abstract
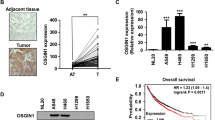
Lung cancer remains a leading cause of cancer-related mortality and morbidity worldwide, of which non-small cell lung cancer (NSCLC) accounts for 80 %. RUVBL1 is a highly conserved eukaryotic AAA+ adenosine 5′-triphosphatase (ATPase) that has many functions highly relevant to cancer. We therefore attempted to determine the potential role of RUVBL1 in the biogenesis of lung adenocarcinoma and obtained some interesting results. Our study revealed that RUVBL1 expression was higher in lung adenocarcinoma specimens than in those of adjacent non-tumor tissues and in lung cancer cell lines than in normal lung cell lines. RUVBL1 knockdown via siRNA reduced proliferation and caused G1/S phase cell cycle arrest in lung adenocarcinoma cell lines. The G1/S phase cell cycle arrest triggered by RUVBL1 downregulation could be attributed, at least in part, to repression of the AKT/GSK-3β/cyclin D1 pathway and probably to the activation of IRE1α-mediated endoplasmic reticulum (ER) stress. We thus demonstrated for the first time that a knockdown of RUVBL1 could effectively inhibit the proliferation of lung adenocarcinoma A549 and H292 cells through the induction of G1/S phase cell cycle arrest via multiple mechanisms. These observations strongly suggested that RUVBL1 should be considered a promising target for the prevention or therapy of lung adenocarcinoma.








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262.
Makino Y, Mimori T, Koike C, Kanemaki M, Kurokawa Y, Inoue S, et al. TIP49, homologous to the bacterial DNA helicase RuvB, acts as an autoantigen in human. Biochem Biophys Res Commun. 1998;245(3):819–23.
Qiu XB, Lin YL, Thome KC, Pian P, Schlegel BP, Weremowicz S, et al. An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J Biol Chem. 1998;273(43):27786–93.
Kurokawa Y, Kanemaki M, Makino Y, Tamura TA. A notable example of an evolutionary conserved gene: studies on a putative DNA helicase TIP49. DNA sequence: the journal of DNA sequencing and mapping. 1999;10(1):37–42.
Kanemaki M, Makino Y, Yoshida T, Kishimoto T, Koga A, Yamamoto K, et al. Molecular cloning of a rat 49-kDa TBP-interacting protein (TIP49) that is highly homologous to the bacterial RuvB. Biochem Biophys Res Commun. 1997;235(1):64–8.
Bao Y, Shen X. SnapShot: chromatin remodeling: INO80 and SWR1. Cell. 2011;144(1):158–e2. doi:10.1016/j.cell.2010.12.024.
Jha S, Shibata E, Dutta A. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol Cell Biol. 2008;28(8):2690–700. doi:10.1128/mcb.01983-07.
Kakihara Y, Houry WA. The R2TP complex: discovery and functions. Biochim Biophys Acta. 2012;1823(1):101–7. doi:10.1016/j.bbamcr.2011.08.016.
Izumi N, Yamashita A, Iwamatsu A, Kurata R, Nakamura H, Saari B, et al. AAA+ proteins RUVBL1 and RUVBL2 coordinate PIKK activity and function in nonsense-mediated mRNA decay. Sci Signal. 2010;3(116):ra27. doi:10.1126/scisignal.2000468.
Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132(6):945–57. doi:10.1016/j.cell.2008.01.019.
Machado-Pinilla R, Liger D, Leulliot N, Meier UT. Mechanism of the AAA+ ATPases pontin and reptin in the biogenesis of H/ACA RNPs. RNA (New York, NY). 2012;18(10):1833–45. doi:10.1261/rna.034942.112.
Gospodinov A, Tsaneva I, Anachkova B. RAD51 foci formation in response to DNA damage is modulated by TIP49. Int J Biochem Cell Biol. 2009;41(4):925–33. doi:10.1016/j.biocel.2008.09.004.
Nano N, Houry WA. Chaperone-like activity of the AAA+ proteins Rvb1 and Rvb2 in the assembly of various complexes. Philos Trans R Soc Lond Ser B Biol Sci. 2013;368(1617):20110399. doi:10.1098/rstb.2011.0399.
Dugan KA, Wood MA, Cole MD. TIP49, but not TRRAP, modulates c-myc and E2F1 dependent apoptosis. Oncogene. 2002;21(38):5835–43. doi:10.1038/sj.onc.1205763.
Bauer A, Huber O, Kemler R. Pontin52, an interaction partner of beta-catenin, binds to the TATA box binding protein. Proc Natl Acad Sci U S A. 1998;95(25):14787–92.
Carlson ML, Wilson ET, Prescott SM. Regulation of COX-2 transcription in a colon cancer cell line by Pontin52/TIP49a. Mol Cancer. 2003;2:42. doi:10.1186/1476-4598-2-42.
Cai Y, Jin J, Yao T, Gottschalk AJ, Swanson SK, Wu S, et al. YY1 functions with INO80 to activate transcription. Nat Struct Mol Biol. 2007;14(9):872–4. doi:10.1038/nsmb1276.
Taniue K, Oda T, Hayashi T, Okuno M, Akiyama T. A member of the ETS family, EHF, and the ATPase RUVBL1 inhibit p53-mediated apoptosis. EMBO Rep. 2011;12(7):682–9. doi:10.1038/embor.2011.81.
Ocak S, Friedman DB, Chen H, Ausborn JA, Hassanein M, Detry B, et al. Discovery of new membrane-associated proteins overexpressed in small-cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9(3):324–36. doi:10.1097/jto.0000000000000090.
Dehan E, Ben-Dor A, Liao W, Lipson D, Frimer H, Rienstein S, et al. Chromosomal aberrations and gene expression profiles in non-small cell lung cancer. Lung Cancer. 2007;56(2):175–84. doi:10.1016/j.lungcan.2006.12.010.
Jha S, Dutta A. RVB1/RVB2: running rings around molecular biology. Mol Cell. 2009;34(5):521–33. doi:10.1016/j.molcel.2009.05.016.
Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1(3):179–86. doi:10.1038/35043058.
Sun Y, Jiang X, Price BD. Tip60: connecting chromatin to DNA damage signaling. Cell cycle (Georgetown, Tex). 2010;9(5):930–6.
D’Amours D, Jackson SP. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3(5):317–27. doi:10.1038/nrm805.
Liu J, Mao Y, Zhang D, Hao S, Zhang Z, Li Z, et al. MiR-143 inhibits tumor cell proliferation and invasion by targeting STAT3 in esophageal squamous cell carcinoma. Cancer Lett. 2016;373(1):97–108. doi:10.1016/j.canlet.2016.01.023.
Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2006;1(1):7–9.
Li XD, Zhang YJ, Han JC. Betulin inhibits lung carcinoma proliferation through activation of AMPK signaling. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(11):11153–8. doi:10.1007/s13277-014-2426-7.
Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, et al. Suppression of c-myc-induced apoptosis by Ras signalling through PI(3) K and PKB. Nature. 1997;385(6616):544–8. doi:10.1038/385544a0.
Zunkler BJ, Wos-Maganga M, Panten U. Fluorescence microscopy studies with a fluorescent glibenclamide derivative, a high-affinity blocker of pancreatic beta-cell ATP-sensitive K+ currents. Biochem Pharmacol. 2004;67(8):1437–44. doi:10.1016/j.bcp.2003.12.011.
Lauscher JC, Elezkurtaj S, Dullat S, Lipka S, Grone J, Buhr HJ, et al. Increased pontin expression is a potential predictor for outcome in sporadic colorectal carcinoma. Oncol Rep. 2012;28(5):1619–24. doi:10.3892/or.2012.1968.
Lacombe J, Mange A, Jarlier M, Bascoul-Mollevi C, Rouanet P, Lamy PJ, et al. Identification and validation of new autoantibodies for the diagnosis of DCIS and node negative early-stage breast cancers. International journal of cancer Journal international du cancer. 2013;132(5):1105–13. doi:10.1002/ijc.27766.
Tung CW, Wu MT, Chen YK, Wu CC, Chen WC, Li HP, et al. Identification of biomarkers for esophageal squamous cell carcinoma using feature selection and decision tree methods. TheScientificWorldJOURNAL. 2013;2013:782031. doi:10.1155/2013/782031.
Wood MA, McMahon SB, Cole MD. An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-myc. Mol Cell. 2000;5(2):321–30.
Bellosta P, Hulf T, Balla Diop S, Usseglio F, Pradel J, Aragnol D, et al. Myc interacts genetically with Tip48/reptin and Tip49/pontin to control growth and proliferation during Drosophila development. Proc Natl Acad Sci U S A. 2005;102(33):11799–804. doi:10.1073/pnas.0408945102.
Etard C, Gradl D, Kunz M, Eilers M, Wedlich D. Pontin and reptin regulate cell proliferation in early Xenopus embryos in collaboration with c-myc and Miz-1. Mech Dev. 2005;122(4):545–56. doi:10.1016/j.mod.2004.11.010.
Nasrabadi D, Larijani MR, Fathi A, Gourabi H, Dizaj AV, Baharvand H, et al. Nuclear proteome analysis of monkey embryonic stem cells during differentiation. Stem Cell Rev. 2010;6(1):50–61. doi:10.1007/s12015-009-9109-6.
Grieb BC, Gramling MW, Arrate MP, Chen X, Beauparlant SL, Haines DS, et al. Oncogenic protein MTBP interacts with MYC to promote tumorigenesis. Cancer Res. 2014;74(13):3591–602. doi:10.1158/0008-5472.can-13-2149.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. doi:10.1016/j.cell.2007.06.009.
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–44. doi:10.1038/nrd2926.
Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23(10):1515–27. doi:10.1016/j.cellsig.2011.05.004.
Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, et al. Dual role of phosphatidylinositol-3, 4, 5-trisphosphate in the activation of protein kinase B. Science (New York, NY). 1997;277(5325):567–70.
Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, et al. 3-phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Current biology: CB. 1997;7(10):776–89.
Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279(4):2737–46. doi:10.1074/jbc.M309999200.
Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7(3):331–42.
Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14(3):2077–86.
Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–511.
Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science (New York, NY). 2011;334(6059):1081–6. doi:10.1126/science.1209038.
Bourougaa K, Naski N, Boularan C, Mlynarczyk C, Candeias MM, Marullo S, et al. Endoplasmic reticulum stress induces G2 cell-cycle arrest via mRNA translation of the p53 isoform p53/47. Mol Cell. 2010;38(1):78–88. doi:10.1016/j.molcel.2010.01.041.
Wang Y, Yu H, Zhang J, Gao J, Ge X, Lou G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer. 2015;15(1):682. doi:10.1186/s12885-015-1706-y.
Lee MR, Lee GH, Lee HY, Kim DS, Chung MJ, Lee YC, et al. BAX inhibitor-1-associated V-ATPase glycosylation enhances collagen degradation in pulmonary fibrosis. Cell Death Dis. 2014;5:e1113. doi:10.1038/cddis.2014.86.
Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, et al. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension. 2014;64(4):738–44. doi:10.1161/hypertensionaha.114.03811.
Shah PP, Beverly LJ. Regulation of VCP/p97 demonstrates the critical balance between cell death and epithelial-mesenchymal transition (EMT) downstream of ER stress. Oncotarget. 2015;6(19):17725–37.
Chen Y, Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23(11):547–55. doi:10.1016/j.tcb.2013.06.005.
Pincus D, Chevalier MW, Aragon T, van Anken E, Vidal SE, El-Samad H, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8(7):e1000415. doi:10.1371/journal.pbio.1000415.
Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–70. doi:10.1016/j.bbamcr.2013.06.028.
Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12(9):440–50. doi:10.1016/j.molmed.2006.07.007.
Xu N, Lao Y, Zhang Y, Gillespie DA. Akt: a double-edged sword in cell proliferation and genome stability. Journal of oncology. 2012;2012:951724. doi:10.1155/2012/951724.
Caporali S, Levati L, Starace G, Ragone G, Bonmassar E, Alvino E, et al. AKT is activated in an ataxia-telangiectasia and Rad3-related-dependent manner in response to temozolomide and confers protection against drug-induced cell growth inhibition. Mol Pharmacol. 2008;74(1):173–83. doi:10.1124/mol.107.044743.
Park J, Feng J, Li Y, Hammarsten O, Brazil DP, Hemmings BA. DNA-dependent protein kinase-mediated phosphorylation of protein kinase B requires a specific recognition sequence in the C-terminal hydrophobic motif. J Biol Chem. 2009;284(10):6169–74. doi:10.1074/jbc.C800210200.
Xipell E, Aragon T, Martinez-Velez N, Vera B, Idoate MA, Martinez-Irujo JJ, et al. Endoplasmic reticulum stress-inducing drugs sensitize glioma cells to temozolomide through downregulation of MGMT, MPG, and Rad51. Neuro-Oncology. 2016. doi:10.1093/neuonc/now022.
Goswami P, Gupta S, Biswas J, Joshi N, Swarnkar S, Nath C, et al. Endoplasmic reticulum stress plays a key role in rotenone-induced apoptotic death of neurons. Mol Neurobiol. 2016;53(1):285–98. doi:10.1007/s12035-014-9001-5.
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J, Fan SJ. Adenovirus-mediated expression of Tob1 sensitizes breast cancer cells to ionizing radiation. Acta Pharmacol Sin. 2007;28(10):1628–36. doi:10.1111/j.1745-7254.2007.00647.x.
Acknowledgments
This study was supported by the National Natural Science Foundation of China, with the grant identification number 31170720.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All specimens were collected after the patients had given their informed consent to participate, and all of the experiments were approved by the institution’s Internal Review and Ethics boards. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflicts of interest
None.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yuan, XS., Wang, ZT., Hu, YJ. et al. Downregulation of RUVBL1 inhibits proliferation of lung adenocarcinoma cells by G1/S phase cell cycle arrest via multiple mechanisms. Tumor Biol. 37, 16015–16027 (2016). https://doi.org/10.1007/s13277-016-5452-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5452-9