Abstract
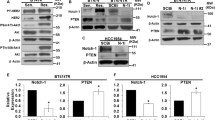
To define the role of the NOTCH signaling pathway in the development of chemoresistance and the associated epithelial–mesenchymal transition (EMT), we investigated the effect of Notch3 on adriamycin (ADM)-resistant human breast cancer cells (MCF-7/ADM cells). We found that Notch3 was downregulated and involved in the chemoresistance of MCF-7/ADM cells, while forced expression of Notch3 reversed the chemoresistance. Furthermore, fos-related antigen 1 (Fra1) was negatively regulated by Notch3 and was highly expressed in MCF-7/ADM cells. Increased Fra1 activated the EMT process. Finally, Notch3 expression was confirmed in clinically chemoresistant samples of breast cancers from patients receiving anthracycline-based chemotherapy. Low expression of Notch3 was an unfavorable predictor of distant relapse-free survival in ER positive breast cancers. Taken together, our findings demonstrate that the Notch3-Fra1 signaling pathway mediates chemoresistance via the EMT.





Similar content being viewed by others
References
Noritaka Y et al. NOTCH3 signaling pathway plays crucial roles in the proliferation of ErbB2-negative human breast cancer cells. Cancer Res. 2008;68:1881–8.
Longley D, Johnston P. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–92.
Gong J, Jaiswal R, Mathys J-M, Combes V, Grau G, Bebawy M. Microparticles and their emerging role in cancer multidrug resistance. Cancer Treat Rev. 2012;38:226–34.
Jäeger W. Classical resistance mechanisms. Int J Clin Pharmacol Ther. 2009;47:46.
Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33.
Jamal M, Chogle SM, Karam SM, Huang GT-J. NOTCH3 is expressed in human apical papilla and in subpopulations of stem cells isolated from the tissue. Genes Dis. 2015;2:261–7.
Farnie G, Clarke RB. Mammary stem cells and breast cancer—role of Notch signalling. Stem Cell Rev. 2007;3:169–75.
Zhang Z et al. Notch3 in human breast cancer cell lines regulates osteoblast-cancer cell interactions and osteolytic bone metastasis. Am J Pathol. 2010;177:1459–69.
Al-Hussaini H, Subramanyam D, Reedijk M, Sridhar SS. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011;10:9–15.
Liu L et al. Notch3 is important for TGF-β-induced epithelial–mesenchymal transition in non-small cell lung cancer bone metastasis by regulating ZEB-1. Cancer Gene Ther. 2014;21:364–72.
Zhang Q et al. Notch3 functions as a regulator of cell self-renewal by interacting with the β-catenin pathway in hepatocellular carcinoma. Oncotarget. 2015;6:3669.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Ohashi S et al. A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res. 2011;71:6836–47.
Thomson S et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non–small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62.
Sabbah M et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–51.
Reisfeld RA. The tumor microenvironment: a target for combination therapy of breast cancer. Crit Rev Oncog. 2013;18:115–33.
Verde P, Casalino L, Talotta F, Yaniv M, Weitzman JB. Deciphering AP-1 function in tumorigenesis: fra-ternizing on target promoters. Cell Cycle. 2007;6:2633–9.
Desmet CJ et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci. 2013;110:5139–44.
Tam WL et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24:347–64.
Shin S, Dimitri CA, Yoon S-O, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38:114–27.
Stinson S et al. TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci Signal. 2011;4:ra41–1.
Belguise K, Milord S, Galtier F, Moquet-Torcy G, Piechaczyk M, Chalbos D. The PKCθ pathway participates in the aberrant accumulation of Fra-1 protein in invasive ER-negative breast cancer cells. Oncogene. 2012;31:4889–97.
Chen H et al. Extracellular signal–regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression. Cancer Res. 2009;69:9228–35.
Ma X et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc Natl Acad Sci. 2012;109:16282–7.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2—ΔΔCT method. Methods. 2001;25:402–8.
He D et al. A new agent developed by biotransformation of polyphyllin VII inhibits chemoresistance in breast cancer. Oncotarget. 2015;7:31814–24.
Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. TGFβ/TNFα-mediated epithelial–mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011;71:4707–19.
Jiang L et al. MiR-489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett. 2014;588:2009–15.
Hatzis C et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305:1873–81.
Park JT, Chen X, Trope CG, Davidson B, Shih I-M, Wang T-L. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am J Pathol. 2010;177:1087–94.
Sayan AE et al. Fra-1 controls motility of bladder cancer cells via transcriptional upregulation of the receptor tyrosine kinase AXL. Oncogene. 2012;31:1493–503.
Acknowledgments
This work was supported by the China National Natural Science Foundation 81572940 and 91439131 to Xin Ma; 31200126 and 31550006 to Dongxu He; 81272358 to Feng Gu; the Natural Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20140004 to Xin Ma); the Program for New Century Excellent Talents in University of The Ministry of Education of China (NCET-12-0880) to Xin Ma; the National High Technology Research and Development Program (863 Program) of China (SQ2015AA020948) to Xin Ma; and the Fundamental Research Funds for the Central Universities (JUSRP51311A, JUSRP51615B and JUSRP51519) to Xin Ma, Dongxu He and Jin Jian. We thank Dr. IC Bruce for reading the manuscript.
Author contributions
Authors who participated in research design are as follows: X.-T. Gu, D.-X. He, X. Ma, and D-Q. Liu. Authors who conducted experiments are as follows: X.-T. Gu, C.-X. Lu, and Y.-F. Lu. Authors who performed data analysis are as follows: X.-T. Gu and D.-X. He. Authors who wrote or contributed to the writing of the manuscript are as follows: X.-T. Gu and D.-X. He.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Xiaoting Gu, Chunxiao Lu, Dongxu He contributed equally to this work.
Electronic supplementary material
Fig. S1
Notch3-Fra1 signaling pathway in MCF-7/PTX. (a) Notch3 expression was lower in MCF-7/PTX (PTX) cells than in MCF-7/WT (WT) cells when analyzed by quantitative real-time PCR (**P < 0.001 versus MCF-7/WT). (b) Notch3 expression was decreased in MCF-7/PTX cells as analyzed by western blotting (∼280 kDa, ***P < 0.0001 versus MCF-7/WT cells). (c) Expression of Fra1 was increased in MCF-7/PTX cells versus MCF-7/WT cells when analyzed by quantitative real-time PCR (***P < 0.0001 versus MCF-7/WT cells). (d) Fra1 expression was decreased in MCF-7/PTX + N3CEG cells as analyzed by western blotting (∼29 kDa, *P < 0.05 versus MCF-7/PTX + pCEG cells). (e) Expression and location of E-cadherin and vimentin in MCF-7/PTX + N3CEG (Notch3 overexpressed in MCF-7/PTX), MCF-7/PTX + pCEG (MCF-7/PTX transfected by an empty plasmid), MCF-7/PTX + siFra1 (Fra1 knocked down in MCF-7/WT), and MCF-7/PTX + NC (MCF-7/PTX transfected by a scrambled siRNA) cells analyzed by confocal microscopy. (b) Cell migration with different treatments (***P < 0.0001 PTX + N3CEG vs PTX + pCEG, ***P < 0.0001 PTX + siFra1 vs PTX + NC). (GIF 143 kb)
Rights and permissions
About this article
Cite this article
Gu, X., Lu, C., He, D. et al. Notch3 negatively regulates chemoresistance in breast cancers. Tumor Biol. 37, 15825–15833 (2016). https://doi.org/10.1007/s13277-016-5412-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5412-4