Abstract
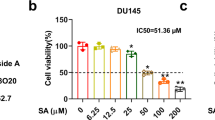
Norcantharidin (NCTD) has an anticancer potential to allow it to be used in the treatment of some malignant cancers. However, whether NCTD may have similar anticancer effects on prostate cancer (PC) is unknown. Here, we aimed to examine the effects of NCTD on PC cells and the underlying mechanisms. We found that NCTD dose-dependently inhibited the PC cell growth, in either a cell counting kit-8 (CCK-8) assay or a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Moreover, NCTD dose-dependently increased the PC cell autophagy, through upregulation of Beclin-1. Furthermore, the Beclin-1 protein, but not mRNA, was regulated by NCTD in PC cells, suggesting post-transcriptional control of Beclin-1 by NCTD. Finally, microRNA (miR)-129-5p was found to be regulated by NCTD, and bioinformatics analyses showed that miR-129-5p targeted the 3′-UTR of Beclin-1 mRNA to inhibit its translation, which was confirmed by luciferase reporter assay. Together, these data suggest that NCTD may upregulate Beclin-1 through suppression of miR-129-5p to induce autophagic cell death and cell proliferation arrest in PC cells. Our study sheds light on using NCTD as a novel treatment for PC.





Similar content being viewed by others
References
Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108:1850–5.
Villar J, Quadri HS, Song I, Tomita Y, Tirado OM, Notario V. PCPH/ENTPD5 expression confers to prostate cancer cells resistance against cisplatin-induced apoptosis through protein kinase calpha-mediated Bcl-2 stabilization. Cancer Res. 2009;69:102–10.
Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A. 2008;105:17356–61.
Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, et al. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol. 2003;39:19–26.
Wang X, Gu Z, Li G, Zhang S, Cao Z, Yang Z, et al. Norcantharidin enhances ABT-263-mediated anticancer activity in neuroblastoma cells by upregulation of noxa. Oncol Rep. 2014;32:716–22.
Shou LM, Zhang QY, Li W, Xie X, Chen K, Lian L, et al. Cantharidin and norcantharidin inhibit the ability of MCF-7 cells to adhere to platelets via protein kinase C pathway-dependent downregulation of alpha2 integrin. Oncol Rep. 2013;30:1059–66.
Lee YC, Lee LM, Yang CH, Lin AM, Huang YC, Hsu CC, et al. Norcantharidin suppresses cell growth and migration with enhanced anticancer activity of gefitinib and cisplatin in human non-small cell lung cancer cells. Oncol Rep. 2013;29:237–43.
Xie X, Wu MY, Shou LM, Chen LP, Gong FR, Chen K, et al. Tamoxifen enhances the anticancer effect of cantharidin and norcantharidin in pancreatic cancer cell lines through inhibition of the protein kinase c signaling pathway. Oncology letters. 2015;9:837–44.
Xie J, Zhang Y, Hu X, Lv R, Xiao D, Jiang L, et al. Norcantharidin inhibits wnt signal pathway via promoter demethylation of WIF-1 in human non-small cell lung cancer. Med Oncol. 2015;32:145.
Chen YJ, Tsai YM, Kuo CD, Ku KL, Shie HS, Liao HF. Norcantharidin is a small-molecule synthetic compound with anti-angiogenesis effect. Life Sci. 2009;85:642–51.
Zhu W, Sun W, Zhang JT, Liu ZY, Li XP, Fan YZ. Norcantharidin enhances TIMP2 antivasculogenic mimicry activity for human gallbladder cancers through downregulating MMP2 and MT1MMP. Int J Oncol. 2015;46:627–40.
Zhang JT, Fan YZ, Chen CQ, Zhao ZM, Sun W. Norcantharidin: a potential antiangiogenic agent for gallbladder cancers in vitro and in vivo. Int J Oncol. 2012;40:1501–14.
Zhang S, Li G, Ma X, Wang Y, Liu G, Feng L, et al. Norcantharidin enhances ABT-737-induced apoptosis in hepatocellular carcinoma cells by transcriptional repression of MCL-1. Cell Signal. 2012;24:1803–9.
Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY, Zhao ZM, et al. Norcantharidin inhibits tumor growth and vasculogenic mimicry of human gallbladder carcinomas by suppression of the PI3-K/MMPS/Ln-5gamma2 signaling pathway. BMC Cancer. 2014;14:193.
Li XP, Jing W, Sun JJ, Liu ZY, Zhang JT, Sun W, et al. A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2,-3 “multi-points priming” mechanisms in vitro and in vivo. BMC Cancer. 2015;15:527.
Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75.
Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–9.
White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–10.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
Huang CY, Huang SP, Lin VC, Yu CC, Chang TY, Lu TL, et al. Genetic variants of the autophagy pathway as prognostic indicators for prostate cancer. Sci Rep. 2015;5:14045.
Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol. 2014;11:508–16.
Shi Y, Han JJ, Tennakoon JB, Mehta FF, Merchant FA, Burns AR, et al. Androgens promote prostate cancer cell growth through induction of autophagy. Mol Endocrinol. 2013;27:280–95.
Ouyang DY, Xu LH, He XH, Zhang YT, Zeng LH, Cai JY, et al. Autophagy is differentially induced in prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing profiles of ATG5. Autophagy. 2013;9:20–32.
Lian J, Karnak D, Xu L. The Bcl-2-Beclin 1 interaction in (−)-gossypol-induced autophagy versus apoptosis in prostate cancer cells. Autophagy. 2010;6:1201–3.
Lamoureux F, Zoubeidi A. Dual inhibition of autophagy and the Akt pathway in prostate cancer. Autophagy. 2013;9:1119–20.
Lin R, Feng J, Dong S, Pan R, Zhuang H, Ding Z. Regulation of autophagy of prostate cancer cells by beta-catenin signaling. Cell Physiol Biochem. 2015;35:926–32.
Sun R, Luo Y, Li J, Wang Q, Li J, Chen X, et al. Ammonium chloride inhibits autophagy of hepatocellular carcinoma cells through SMAD2 signaling. Tumour Biol. 2015;36:1173–7.
Mei Q, Li F, Quan H, Liu Y, Xu H. Busulfan inhibits growth of human osteosarcoma through miR-200 family microRNAs in vitro and in vivo. Cancer Sci. 2014;105:755–62.
Chang Y, Zhao Y, Gu W, Cao Y, Wang S, Pang J, et al. Bufalin inhibits the differentiation and proliferation of cancer stem cells derived from primary osteosarcoma cells through miR-148a. Cell Physiol Biochem. 2015;36:1186–96.
Song W, Li Q, Wang L, Wang L. Modulation of FOXO1 expression by miR-21 to promote growth of pancreatic ductal adenocarcinoma. Cell Physiol Biochem. 2015;35:184–90.
Jin Y, Lu J, Wen J, Shen Y, Wen X. Regulation of growth of human bladder cancer by miR-192. Tumour Biol. 2015;36:3791–7.
Wang Q, Cai J, Wang J, Xiong C, Zhao J. miR-143 inhibits EGFR-signaling-dependent osteosarcoma invasion. Tumour Biol. 2014;35:12743–8.
Liu G, Jiang C, Li D, Wang R, Wang W. miRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in gastric cancer. Tumour Biol. 2014;35:9801–6.
Wang F, Xiao W, Sun J, Han D, Zhu Y. miRNA-181c inhibits EGFR-signaling-dependent MMP9 activation via suppressing Akt phosphorylation in glioblastoma. Tumour Biol. 2014;35:8653–8.
Huang SQ, Liao QJ, Wang XW, Xin DQ, Chen SX, Wu QJ, et al. RNAi-mediated knockdown of pituitary tumor- transforming gene-1 (PTTG1) suppresses the proliferation and invasive potential of PC3 human prostate cancer cells. Braz J Med Biol Res. 2012;45:995–1001.
Coronnello C, Benos PV. ComiR: combinatorial microRNA target prediction tool. Nucleic Acids Res. 2013;41:W159–64.
Brest P, Lassalle S, Hofman V, Bordone O, Gavric Tanga V, Bonnetaud C, et al. miR-129-5p is required for histone deacetylase inhibitor-induced cell death in thyroid cancer cells. Endocr Relat Cancer. 2011;18:711–9.
Duan L, Hao X, Liu Z, Zhang Y, Zhang G. miR-129-5p is down-regulated and involved in the growth, apoptosis and migration of medullary thyroid carcinoma cells through targeting RET. FEBS Lett. 2014;588:1644–51.
Li M, Tian L, Wang L, Yao H, Zhang J, Lu J, et al. Down-regulation of miR-129-5p inhibits growth and induces apoptosis in laryngeal squamous cell carcinoma by targeting APC. PLoS One. 2013;8, e77829.
Dossing KB, Binderup T, Kaczkowski B, Jacobsen A, Rossing M, Winther O, et al. Down-regulation of miR-129-5p and the let-7 family in neuroendocrine tumors and metastases leads to up-regulation of their targets Egr1, G3bp1, Hmga2 and Bach1. Genes (Basel). 2014;6:1–21.
Long XH, Zhou YF, Peng AF, Zhang ZH, Chen XY, Chen WZ, et al. Demethylation-mediated miR-129-5p up-regulation inhibits malignant phenotype of osteogenic osteosarcoma by targeting Homo sapiens valosin-containing protein (VCP). Tumour Biol. 2015;36:3799–806.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Xiao, W., Dai, B., Zhu, Y. et al. Norcantharidin induces autophagy-related prostate cancer cell death through Beclin-1 upregulation by miR-129-5p suppression. Tumor Biol. 37, 15643–15648 (2016). https://doi.org/10.1007/s13277-015-4488-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4488-6