Abstract
Radiotherapy is widely used in the treatment of nasopharyngeal carcinoma (NPC), whereas its effects on the NPC growth, survival, and metastases have not been completely evaluated. Here, we compared the detected metastatic NPC tissues after radiotherapy (m-NPC) to the resected primary NPC tissues prior to radiotherapy (p-NPC). We detected higher levels of Snail2 protein, but not mRNA in m-NPC, compared to p-NPC. In vitro, a modest irradiation on NPC cells resulted in significant cell death, but increased Snail2 protein, but mRNA levels in the surviving NPC cells. Bioinformatics analyses showed that miR-613, which was significantly decreased in NPC cells after irradiation, targeted the 3′-UTR of Snail2 mRNA to inhibit its translation. Moreover, miR-613 overexpression inhibited Snail2-mediated cell invasiveness, while miR-613 depletion increased Snail2-mediated cell invasiveness in NPC cells. Finally, we detected significantly lower levels of miR-613 in m-NPC, compared to p-NPC. Together our data suggest that although radiotherapy induced NPC cell death, it may increase Snail2-mediated NPC cell invasiveness through downregulating miR-613.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is the most prevalent malignant nasopharyngeal tumor, with the highest incidence detected in southern China [1–3]. Due to the specific location and asymptomatic features, most NPC subjects fail to be diagnosed at an early stage and thus miss the best time course for an optimized treatment [1–3]. Radiation therapy or radiotherapy uses high-energy X-rays or particles to either eliminate cancer cells or impair their growth [4–6]. Radiotherapy has been commonly applied in the NPC treatment since most of NPC cases are very sensitive to irradiation [4–6]. However, the effects of radiotherapy may be paradoxical since radiotherapy induces a complex reaction in cancer cells and affects many genes related to NPC cell growth, survival, and metastases [1–3].
Zinc finger protein Snail2 is an important member from the Snail family of C2H2-type zinc finger transcription factors and is encoded by the SNAI2 gene in human [7, 8]. In many types of cancer cells, Snail2 is a potent transcriptional repressor that represses transcription of E-cadherin through E-box-motif bindings. Hence, Snail2 potently triggers an epithelial–mesenchymal transition (EMT) process to allow cancer cells to invade, outgrow, and migrate [9–13].
MicroRNA (miRNA) is a class of 18–23-nucleotide non-coding small RNAs that regulate gene expression at translational level through their base-pairing to the 3′-untranslated region (3′-UTR) of target mRNAs [14, 15]. It has been acknowledged that miRNAs regulate many biological events and specifically play a critical role during carcinogenesis of various cancers [16–18], including NPC [19–22]. Among all miRNAs, miR-613 was mainly studied in lipid metabolism [23–26]. Recently, a possible involvement of miR-613 has been suggested in regulating aggressive papillary thyroid carcinoma [27]. However, whether miR-613 may similarly regulate the metastases of NPC has not been reported.
In this study, we compared the detected metastatic NPC tissues after radiotherapy (m-NPC) to the resected primary NPC tissues prior to radiotherapy (p-NPC). We detected higher levels of Snail2 protein, but not mRNA in m-NPC, compared to p-NPC. In vitro, a modest irradiation on NPC cells resulted in significant cell death, but increased Snail2 protein, but mRNA levels in the surviving NPC cells. Bioinformatics analyses showed that miR-613, which was significantly decreased in NPC cells after irradiation, targeted the 3′-UTR of Snail2 mRNA to inhibit its translation. Moreover, miR-613 overexpression inhibited Snail2-mediated cell invasiveness, while miR-613 depletion increased Snail2-mediated cell invasiveness in NPC cells. Finally, we detected significantly lower levels of miR-613 in m-NPC, compared to p-NPC. Together our data suggest that although radiotherapy induced NPC cell death, it may increase Snail2-mediated NPC cell invasiveness through downregulating miR-613.
Materials and methods
Patient tissue specimens
A total of 25 specimens from NPC patients were collected for this study. Metastatic NPC tissues after radiotherapy (m-NPC) and the paired resected primary NPC tissues prior to radiotherapy (p-NPC) were analyzed and compared to each other. All specimens had been histologically and clinically diagnosed at the Department of Radiation Oncology of Quzhou People Hospital from 2009 to 2014. For the use of these clinical materials for research purposes, prior patient's consents and approval from the Institutional Research Ethics Committee were obtained.
Human NPC cell line culture, transfection, and irradiation
All NPC cell lines in the current study were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). NPC cell lines CNE-1 [28], CNE-2, 5-8F, and 6-10B were all maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA). All cell lines were incubated in a humidified chamber with 5 % CO2 at 37 °C. Since results using different cell lines were similar, only data of CNE-1 cells were shown. MiR-613-expressing and antisense (as) plasmids were prepared with a general method. The NPC cells were also transfected with a null plasmid as a control (null). Transfection was performed with Lipofectamine 2000 reagent (Invitrogen), according to the instructions of the manufacturer. Transfected cells expressing miR-613, or as-miR-613, or control null were purified by flow cytometry based on GFP. For irradiation of the cell lines, the plated cells were exposed to 2 Gy irradiation for 24 h and then analyzed.
Cell viability by cell counting kit-8 (CCK-8) assay
The CCK-8 detection kit (Sigma-Aldrich) was used to measure cell viability according to the manufacturer’s instructions. Briefly, cells were seeded in a 96-well microplate at a density of 5×104/ml. After 24 h, cells were treated with resveratrol. Subsequently, CCK-8 solution (20 ml/well) was added, and the plate was incubated at 37 °C for 2 h. The viable cells were counted by absorbance measurements with a monochromator microplate reader at a wavelength of 450 nm. The optical density value was reported as the percentage of cell viability in relation to the control group (set as 100 %).
Apoptosis assay by flow cytometry
For analyses of cell apoptosis, the dissociated tissue cells or cultured cells were re-suspended at a density of 106 cells/ml in PBS. After double staining with FITC-Annexin V and propidium iodide (PI) from a FITC Annexin V Apoptosis Detection Kit I (Becton-Dickinson Biosciences, San Jose, CA, USA), cells were analyzed using a FACScan flow cytometer (Becton-Dickinson Biosciences) equipped with Cell Quest software (Becton-Dickinson Biosciences) for determination of Annexin V+ PI-apoptotic cells.
Western blot
The protein was extracted from the NPC tissue or cultured NPC cell lines with RIPA lysis buffer (1 % NP40, 0.1 % SDS, 100 μg/ml phenylmethylsulfonyl fluoride, 0.5 % sodium deoxycholate, in PBS) on ice. The supernatants were collected after centrifugation at 12,000 ×g at 4 °C for 20 min. Protein concentration was determined using a BCA protein assay kit (Bio-rad, China), and whole lysates were mixed with 4 × SDS loading buffer (125 mmol/l Tris–HCl, 4 % SDS, 20 % glycerol, 100 mmol/l DTT, and 0.2 % bromophenol blue) at a ratio of 1:3. Samples were heated at 100 °C for 5 min and were separated on SDS-polyacrylamide gels. The separated proteins were then transferred to a PVDF membrane. The membrane blots were first probed with a primary antibody. After incubation with horseradish peroxidase-conjugated second antibody, autoradiograms were prepared using the enhanced chemiluminescent system to visualize the protein antigen. The signals were recorded using an X-ray film. Primary antibodies were rabbit anti-Snail2 and anti-α-tubulin (Cell Signaling, San Jose, CA, USA). Secondary antibody is HRP-conjugated anti-rabbit (Jackson ImmunoResearch Labs, West Grove, PA, USA). Blotting images were representative from five repeats. α-Tubulin was used as a protein loading control.
RT-qPCR
miRNA and total RNA were extracted from NPC specimens or from cultured cells with miRNeasy mini kit or RNeasy kit (Qiagen, Hilden, Germany) for cDNA synthesis. Complementary DNA (cDNA) was randomly primed from 2 μg of total RNA using the Omniscript reverse transcription kit (Qiagen). Real-time quantitative PCR (RT-qPCR) was subsequently performed in duplicates with QuantiTect SYBR Green PCR Kit (Qiagen). All primers were purchased from Qiagen. Data were collected and analyzed using 2-△△Ct method for quantification of the relative mRNA expression levels. Values of genes were first normalized against α-tubulin and then compared to controls.
Luciferase-reporter activity assay
Luciferase-reporters were successfully constructed using molecular cloning technology. Target sequence for Snail2 miRNA 3′-UTR clone was purchased from Creative Biogene (Shirley, NY, USA). MiR-613-modified CNE-1 cells were seeded in 24-well plates for 24 h, after which they were transfected with 1 μg of luciferase-reporter plasmids per well. Luciferase activities were measured using the dual-luciferase reporter gene assay kit (Promega, Beijing, China), according to the manufacturer’s instructions.
Transwell cell migration assay
Cells (104) were plated into the top side of polycarbonate transwell filter coated with Matrigel in the upper chamber of the BioCoatTM Invasion Chambers (Becton-Dickinson Biosciences, Bedford, MA, USA) and incubated at 37 °C for 22 h. The cells inside the upper chamber with cotton swabs were then removed. Migratory and invasive cells on the lower membrane surface were fixed, stained with hematoxylin, and counted for ten random ×100 fields per well. Cell counts are expressed as the mean number of cells per field of view. Five independent experiments were performed, and the data are presented as mean ± standard deviation (SD).
Statistical analysis
All statistical analyses were carried out using the SPSS 17.0 statistical software package. All values are depicted as mean ± standard deviation (SD) and are considered significant if p < 0.05. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s exact test for comparison of two groups.
Results
Metastatic NPC tissue contains higher levels of Snail2 protein compared to primary PNC tissue
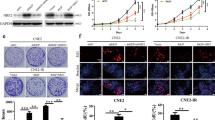
We compared the detected metastatic NPC tissues after radiotherapy (m-NPC) to the resected primary NPC tissues prior to radiotherapy (p-NPC). We detected higher levels of Snail2 protein (Fig. 1a), but not mRNA (Fig. 1b) in m-NPC, compared to p-NPC. These data suggest that the metastatic potential of NPC may increase after the cancer cells are exposed to irradiation, and this change in cancer cell invasiveness may result from the post-transcriptional control of Snail2.
Metastatic NPC tissue contains higher levels of Snail2 protein compared to primary PNC tissue. a, b We compared the detected metastatic NPC tissues after radiotherapy (m-NPC) to the resected primary NPC tissues prior to radiotherapy (p-NPC). We detected higher levels of Snail2 protein (a), but not mRNA (b) in m-NPC, compared to p-NPC. *p < 0.05. NS non-significant. N = 25
Irradiation on NPC cells increases apoptosis-associated cell death in vitro
Then, we tested the effects of irradiation on NPC cells in vitro. Irradiation at 2 Gy was given to human NPC cell line CNE-1. We found that irradiation induced significant decreases in cell number in a CKK-8 assay (Fig. 2a), resulting from a significant increase in cell apoptosis, shown by quantification (Fig. 2b) and by representative flow charts (Fig. 2c). These data confirm that irradiation on NPC cells increases apoptosis-associated cell death in vitro.
Irradiation on NPC cells increases apoptosis-associated cell death in vitro. a–c We tested the effects of irradiation on NPC cells in vitro. Irradiation at 2 Gy was given to human NPC cell line CNE-1. We found that irradiation induced significant decreases in cell number in a CKK-8 assay (a), resulting from a significant increase in cell apoptosis, shown by quantification (b) and by representative flow charts (c). *p < 0.05. N = 5
Irradiation on NPC cells increases Snail2 protein, but mRNA levels in the surviving NPC cells
Next, we examined the effects of irradiation on Snail2 expression in CNE-1 cells. We found that irradiation significantly increased Snail2 protein (Fig. 3a), but mRNA levels (Fig. 3b) in the surviving NPC cells. These data are consistent with clinical findings and suggest that the metastatic potential of NPC cells may increase after the cancer cells are exposed to irradiation, and this change in cancer cell invasiveness may result from the post-transcriptional control of Snail2.
Irradiation on NPC cells increases Snail2 protein, but mRNA levels in the surviving NPC cells. a, b We examined the effects of irradiation on Snail2 expression in CNE-1 cells. We found that irradiation significantly increased Snail2 protein (a), but mRNA levels (b) in the surviving NPC cells. *p < 0.05. NS non-significant. N = 5
MiR-613 decreases in the irradiated NPC cells and miR-613 targets 3′-UTR of Snail2 mRNA to inhibit its expression
Since our data suggest the presence of a post-transcriptional regulation of Snail2 induced by irradiation, we hypothesized that irradiation may alter the expression of a Snail2-targeting miRNA. We then checked all Snail2-targeting miRNAs to see which one was affected by irradiation. Specifically, we found that miR-613 targeted Snail2 mRNA by bioinformatics analyses, showing that miR-613 binds to 3′-UTR of Snail2 mRNA from 671th to 6796th base site (Fig. 4a). Moreover, irradiation significantly reduced the levels of miR-613 in NPC cells (Fig. 4b). In order to examine whether miR-613 may regulate Snail2 in NPC cells, we either overexpressed miR-613 or inhibited miR-613 in CNE-1 cells by transfection of the cells with a miR-613-expressing plasmid (CNE-1-miR-613) or with a plasmid carrying miR-613 antisense (CNE-1-as-miR-613). The CNE-1 cells were also transfected with a null plasmid as a control (CNE-1-null). Co-expression of a GFP reporter in these plasmids allows the purification of transfected cells by flow cytometry. The overexpression or inhibition of miR-613 in NPC cells was confirmed by RT-qPCR (Fig. 4c). MiR-613-modified CNE-1 cells were then transfected with 1 μg of Snail2-3′-UTR luciferase-reporter plasmid. The luciferase activities were quantified in these cells, suggesting that miR-613 targets the 3′-UTR of Snail2 mRNA to inhibit its translation (Fig. 4d).
MiR-613 decreases in the irradiated NPC cells and miR-613 targets 3′-UTR of Snail2 mRNA to inhibit its expression. a Bioinformatics analyses show that miR-613 binds to 3′-UTR of Snail2 mRNA from 671th to 6796th base site. b Irradiation significantly reduced the levels of miR-613 in NPC cells. c, d In order to examine whether miR-613 may regulate Snail2 in NPC cells, we either overexpressed miR-613 or inhibited miR-613 in CNE-1 cells by transfection of the cells with a miR-613-expressing plasmid (CNE-1-miR-613) or with a plasmid carrying miR-613 antisense (CNE-1-as-miR-613). The CNE-1 cells were also transfected with a null plasmid as a control (CNE-1-null). Co-expression of a GFP reporter in these plasmids allows purification of transfected cells by flow cytometry. The overexpression or inhibition of miR-613 in NPC cells was confirmed by RT-qPCR (c). MiR-613-modified CNE-1 cells were then transfected with 1 μg of Snail2-3′-UTR luciferase-reporter plasmid. The luciferase activities were quantified in these cells, suggesting that miR-613 targets 3′-UTR of Snail2 mRNA to inhibit its translation (d). *p < 0.05. N = 5
MiR-613 inhibits Snail2 in NPC cells
We found that alteration of miR-613 in CNE-1 cells did not change the mRNA levels of Snail2 (Fig. 5a). However, overexpression of miR-613 significantly decreased Snail2 protein levels in CNE-1 cells, while inhibition of miR-613 significantly increased Snail2 protein levels in CNE-1 cells by Western blot (Fig. 5b). These data suggest that MiR-613 inhibits Snail2 protein translation in CNE-1 cells.
MiR-613 inhibits Snail2 in NPC cells. a Alteration of miR-613 in CNE-1 cells did not change the mRNA levels of Snail2. b Overexpression of miR-613 significantly decreased Snail2 protein levels in CNE-1 cells, while inhibition of miR-613 significantly increased Snail2 protein levels in CNE-1 cells by Western blot. *p < 0.05. NS non-significant. N = 5
MiR-613 inhibits Snail2-mediated NPC cell invasiveness
We found that overexpression of miR-613 resulted in decreases in cell invasiveness of CNE-1 cells in a transwell cell migration assay, shown by quantification (Fig. 6a) and by representative images (Fig. 6b). Similarly, depletion of miR-613 resulted in increases in cell invasiveness of CNE-1 cells in a transwell cell migration assay, shown by quantification (Fig. 6a) and by representative images (Fig. 6b). Thus, miR-613 inhibits Snail2-mediated NPC cell invasiveness.
MiR-613 inhibits Snail2-mediated NPC cell invasiveness. a, b Overexpression of miR-613 resulted in decreases in cell invasiveness of CNE-1 cells in a transwell cell migration assay, shown by quantification (a) and by representative images (b). Similarly, depletion of miR-613 resulted in increases in cell invasiveness of CNE-1 cells in a transwell cell migration assay, shown by quantification (a) and by representative images (b). *p < 0.05. NS non-significant. N = 5
Metastatic NPC tissue contains lower levels of miR-613 compared to primary PNC tissue
Finally, we compared the levels of miR-613 between m-NPC and p-NPC and found that m-NPC had significantly lower levels of miR-613, compared to p-NPC (Fig. 7). Together our data suggest that although radiotherapy induced NPC cell death, it may increase Snail2-mediated NPC cell invasiveness through downregulating miR-613.
Discussion
Many miRNAs play important roles in the invasion and metastasis of NPC cells. However, the relationship among radiotherapy, miRNA expression, and EMT-associate proteins has not been established in NPC. In this study, we addressed these questions.
By sequence matching, we found a number of candidate miRNAs that target Snail2, including miR-1, miR-203, miR-30, miR-384-5p, miR-124, miR-613, miR-206, miR-613, etc. Among all these miRNAs, we specifically detected a significant decrease in miR-613 and a significant increase in Snail2 protein in metastatic NPC specimens after irradiation, compared to the paired primary NPC tissue. Similarly, these data were reproduced in vitro in several NPC cell lines, showing that irradiation has a similar effect on miR-613 and Snail2. Overexpression of miR-613 inhibited cell invasion, while depletion of miR-133 increased cell invasion. Moreover, in miR-613-modified NPC cells, we found that the alteration in miR-613 levels did not affect Snail2 mRNA, but regulated the protein level. By promoter luciferase assay, we concluded that miR-613 inhibited Snail2 through translation suppression. Further, miR-613-suppressed Snail2 levels, resulting in changes in cell invasiveness.
Hence, although irradiation may eliminate a great number of cancer cells, the surviving cells may increase Snail2 levels through downregulating miR-613-mediated protein translation suppression. These mechanisms may increase the invasiveness of the cancer cells, resulting in augmented cancer cell metastases. Therefore, approaches may be taken to contradict this mechanism in NPC cells after radiotherapy to substantially reduce cancer cell migration and recurrence.
To summarize, we propose a novel model that regulates NPC metastases post-radiotherapy. Irradiation eliminates majority of cancer cells but increases surviving cell invasion through downregulation of miR-613, which is required to suppress Snail2 protein translation. Thus, our study highlights miR-613 as a promising novel target to be treated during NPC radiotherapy.
References
Zhang L, Yang L, Li JJ, Sun L. Potential use of nucleic acid-based agents in the sensitization of nasopharyngeal carcinoma to radiotherapy. Cancer Lett. 2012;323:1–10.
King AD, Ahuja AT, Yeung DK, Wong JK, Lee YY, Lam WW, et al. Delayed complications of radiotherapy treatment for nasopharyngeal carcinoma: imaging findings. Clin Radiol. 2007;62:195–203.
Lee AW. Contribution of radiotherapy to function preservation and cancer outcome in primary treatment of nasopharyngeal carcinoma. World J Surg. 2003;27:838–43.
Zhang T, Sun Q, Liu T, Chen J, Du S, Ren C, et al. Mir-451 increases radiosensitivity of nasopharyngeal carcinoma cells by targeting ras-related protein 14 (rab14). Tumour Biol. 2014;35:12593–9.
Win KT, Lee SW, Huang HY, Lin LC, Lin CY, Hsing CH, et al. Nicotinamide n-methyltransferase overexpression is associated with akt phosphorylation and indicates worse prognosis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34:3923–31.
Kao YC, Lee SW, Lin LC, Chen LT, Hsing CH, Hsu HP, et al. Fatty acid synthase overexpression confers an independent prognosticator and associates with irradiation resistance in nasopharyngeal carcinoma. Tumour Biol. 2013;34:759–68.
Li W, Jiang G, Zhou J, Wang H, Gong Z, Zhang Z, et al. Down-regulation of mir-140 induces emt and promotes invasion by targeting slug in esophageal cancer. Cell Physiol Biochem. 2014;34:1466–76.
Qiu YH, Wei YP, Shen NJ, Wang ZC, Kan T, Yu WL, et al. Mir-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell Physiol Biochem. 2013;32:1331–41.
Niu H, Wu B, Jiang H, Li H, Zhang Y, Peng Y, et al. Mechanisms of rhogdi2 mediated lung cancer epithelial–mesenchymal transition suppression. Cell Physiol Biochem. 2014;34:2007–16.
Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang X, et al. Mir-146b-5p promotes metastasis and induces epithelial–mesenchymal transition in thyroid cancer by targeting znrf3. Cell Physiol Biochem. 2015;35:71–82.
Guo J, Xia N, Yang L, Zhou S, Zhang Q, Qiao Y, et al. Gsk-3beta and vitamin d receptor are involved in beta-catenin and snail signaling in high glucose-induced epithelial–mesenchymal transition of mouse podocytes. Cell Physiol Biochem. 2014;33:1087–96.
Teng Y, Zhao L, Zhang Y, Chen W, Li X. Id-1, a protein repressed by mir-29b, facilitates the tgfbeta1-induced epithelial–mesenchymal transition in human ovarian cancer cells. Cell Physiol Biochem. 2014;33:717–30.
Yang T, Chen M, Sun T. Simvastatin attenuates tgf-beta1-induced epithelial–mesenchymal transition in human alveolar epithelial cells. Cell Physiol Biochem. 2013;31:863–74.
Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev. 2013;23:3–11.
Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2013;18:282–9.
Mei Q, Li F, Quan H, Liu Y, Xu H. Busulfan inhibits growth of human osteosarcoma through mir-200 family microRNAs in vitro and in vivo. Cancer Sci. 2014;105:755–62.
Wang F, Xiao W, Sun J, Han D, Zhu Y. Mirna-181c inhibits egfr-signaling-dependent mmp9 activation via suppressing akt phosphorylation in glioblastoma. Tumour Biol. 2014;35:8653–8.
Liu G, Jiang C, Li D, Wang R, Wang W. Mirna-34a inhibits egfr-signaling-dependent mmp7 activation in gastric cancer. Tumour Biol. 2014;35:9801–6.
Qi X, Li J, Zhou C, Lv C, Tian M. Mir-142-3p suppresses socs6 expression and promotes cell proliferation in nasopharyngeal carcinoma. Cell Physiol Biochem. 2015;36:1743–52.
Xie M, Yi X, Wang R, Wang L, He G, Zhu M, et al. 14-Thienyl methylene matrine (yyj18), the derivative from matrine, induces apoptosis of human nasopharyngeal carcinoma cells by targeting mapk and pi3k/akt pathways in vitro. Cell Physiol Biochem. 2014;33:1475–83.
Yang X, Ni W, Lei K. Mir-200b suppresses cell growth, migration and invasion by targeting notch1 in nasopharyngeal carcinoma. Cell Physiol Biochem. 2013;32:1288–98.
Zhang SX, Qiu QH, Chen WB, Liang CH, Huang B. Celecoxib enhances radiosensitivity via induction of g(2)-m phase arrest and apoptosis in nasopharyngeal carcinoma. Cell Physiol Biochem. 2014;33:1484–97.
Zhao R, Feng J, He G. Mir-613 regulates cholesterol efflux by targeting lxralpha and abca1 in ppargamma activated thp-1 macrophages. Biochem Biophys Res Commun. 2014;448:329–34.
Sacco J, Adeli K. MicroRNAs: emerging roles in lipid and lipoprotein metabolism. Curr Opin Lipidol. 2012;23:220–5.
Zhong D, Zhang Y, Zeng YJ, Gao M, Wu GZ, Hu CJ, et al. Microrna-613 represses lipogenesis in hepg2 cells by downregulating lxralpha. Lipids Health Dis. 2013;12:32.
Ou Z, Wada T, Gramignoli R, Li S, Strom SC, Huang M, et al. MicroRNA hsa-mir-613 targets the human lxralpha gene and mediates a feedback loop of lxralpha autoregulation. Mol Endocrinol. 2011;25:584–96.
Yang Z, Yuan Z, Fan Y, Deng X, Zheng Q. Integrated analyses of microRNA and mRNA expression profiles in aggressive papillary thyroid carcinoma. Mol Med Rep. 2013;8:1353–8.
Establishment of an epitheloid cell line and a fusiform cell line from a patient with nasopharyngeal carcinoma. Sci Sin 1978;21:127–134.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Zheng Peng and Tiancai Xu contributed equally to this work.
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-017-5487-6.
About this article
Cite this article
Peng, Z., Xu, T., Liao, X. et al. RETRACTED ARTICLE: Effects of radiotherapy on nasopharyngeal carcinoma cell invasiveness. Tumor Biol. 37, 15559–15566 (2016). https://doi.org/10.1007/s13277-015-3960-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3960-7