Abstract
Backgrounds
Fisetin (tetrahydroxyflavone), a natural plant component, is known to have different properties including, direct radical scavenger, anti-inflammation, and cell survival.
Methods
We investigated whether fisetin can protect cardiomyocytes against H2O2-induced apoptosis that is relevant to cardiovascular disease risks using cell viability test, ROS measurement, and Western blotting.
Results
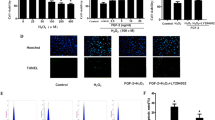
Fisetin decreased apoptotic cell death and intracellular reactive oxygen species (ROS), while enhancing the expression of Cu/Zn-superoxide dismutase (SOD) as well as phosphorylation of Akt and extracellular regulated kinase (ERK) 1/2. In particular, fisetin significantly decreased apoptosis through inhibition of cleaved caspase-3 and Bax expression and by enhancing the expression of anti-apoptotic enzyme as well as Bcl-2 in H2O2-stimulated H9c2 cells. However, the expression of heme oxygenase, catalase, and Mn-SOD was not altered by fisetin in these conditions.
Conclusion
Taken together, these data suggest that the potential cardioprotective effect of fisetin may involve Cu/Zn-SOD-mediated activation of Bcl-2 through the Akt and ERK1/2 pathways.
Similar content being viewed by others
References
Gada, H., Agarwal, S. & Marwick, T. H. Perspective on the cost-effectiveness of transapical aortic valve implantation in high-risk patients: Outcomes of a decision-analytic model. Ann Cardiothoracic Surg 1, 145–155 (2012).
Jin, C. N. et al. The prevalence and prognosis of resistant hypertension in patients with heart failure. PLoS One 9, e114958 (2014).
Mampuya, W. M. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2, 38–49 (2012).
Konstantinidis, K., Whelan, R. S. & Kitsis, R. N. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol 32, 1552–1562 (2012).
Feng, Y., Wang, Y., Cao, N., Yang, H. & Wang, Y. Progenitor/stem cell transplantation for repair of myocardial infarction: Hype or hope? Ann Palliat Med 1, 65–77 (2012).
Fujita, T. & Ishikawa, Y. Apoptosis in heart failure. -The role of the beta-adrenergic receptor-mediated signaling pathway and p53-mediated signaling pathway in the apoptosis of cardiomyocytes. Circ J 75, 1811–1818 (2011).
Kung, G., Konstantinidis, K. & Kitsis, R. N. Programmed necrosis, not apoptosis, in the heart. Circ Res 108, 1017–1036 (2011).
Xu, F., Yu, H., Liu, J. & Cheng, L. alphaB-crystallin regulates oxidative stress-induced apoptosis in cardiac H9c2 cells via the PI3K/AKT pathway. Mol Biol Rep 40, 2517–2526 (2013).
Lamendola, P. et al. Mechanisms of myocardial cell protection from ischemia/reperfusion injury and potential clinical implications. G Ital Cardiol (Rome) 10, 28–36 (2009).
Lee, Y. S. et al. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemia-reperfusion injury. Apoptosis 11, 1091–1100 (2006).
Halestrap, A. P., Clarke, S. J. & Javadov, S. A. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61, 372–385 (2004).
Zhu, X. et al. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol 293, H1442–450 (2007).
Arai, Y. et al. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130, 2243–2250 (2000).
Ishige, K., Schubert, D. & Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30, 433–446 (2001).
Fotsis, T. et al. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin Endocrinol Metab 12, 649–666 (1998).
Sung, B., Pandey, M. K. & Aggarwal, B. B. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol 71, 1703–1714 (2007).
Hanneken, A., Lin, F. F., Johnson, J. & Maher, P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci 47, 3164–3177 (2006).
Wang, L. et al. Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem 54, 9798–9804 (2006).
MacLellan, W. R. & Schneider, M. D. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res 81, 137–144 (1997).
Liu, H., Li, X., Qin, F. & Huang, K. Selenium suppresses oxidative-stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplasmic reticulum stress. J Biol Inorg Chem 19, 375–388 (2014).
Michiels, C., Raes, M., Toussaint, O. & Remacle, J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17, 235–248 (1994).
Amaravadim, R. & Thompsonm, C. B. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 115, 2618–2624 (2005).
Turner, N. A. et al. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J Mol Cell Cardiol 30, 1789–1801 (1998).
Liu, J. et al. Recombinant PTD-Cu/Zn SOD attenuates hypoxia-reoxygenation injury in cardiomyocytes. Free Radic Res 47, 386–393 (2013).
Zhong, W. et al. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J Steroid Biochem Mol Biol 140, 56–62 (2013).
Nijveldt, R. J. et al. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74, 418–425 (2001).
Khan, N., Syed, D. N., Ahmad, N. & Mukhtar, H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19, 151–162 (2013).
Kowaltowski, A. J., de Souza-Pinto, N. C., Castilho, R. F. & Vercesi, A. E. Mitochondria and reactive oxygen species. Free Radic Biol Med 47, 333–343 (2009).
Ali, M. A., Kandasamy, A. D., Fan, X. & Schulz, R. Hydrogen peroxide-induced necrotic cell death in cardiomyocytes is independent of matrix metalloproteinase-2. Toxicol In Vitro 27, 1686–1692 (2013).
Chandra, J., Samali, A. & Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29, 323–333 (2000).
Han, H. et al. Progressive apoptotic cell death triggered by transient oxidative insult in H9c2 rat ventricular cells: a novel pattern of apoptosis and the mechanisms. Am J Physiol Heart Circ Physiol 286, H2169–2182 (2004).
Back, O. C. et al. The Protective Effect of INH2BP, a Novel PARP Inhibitor 5-Iodo-6-Amino-1,2-Benzopyrone, Against Hydrogen Peroxide-Induced Apoptosis Through ERK and p38 MAPK in H9c2 Cells. Pharmacology 96, 259–270 (2015).
Clement, M. V., Ponton, A. & Pervaiz, S. Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. FEBS Lett 440, 13–18 (1998).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, J.S., Lee, JS., Cha, K.J. et al. Fisetin protects H9c2 cardiomyoblast cells against H2O2-induced apoptosis through Akt and ERK1/2 signaling pathways. Mol. Cell. Toxicol. 14, 183–192 (2018). https://doi.org/10.1007/s13273-018-0020-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-018-0020-6