Abstract
Backgrounds
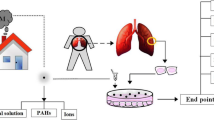
Exposure to airborne particulate matter (PM2.5), a PM with an aerodynamic diameter of less than 2.5 μm, is known to be associated with a variety of adverse health effects, particularly related to the respiratory system. However, the molecular mechanisms involved in fine PM toxicity are still not well-characterized. In this study, we estimate pulmonary toxic mechanism using two types (water soluble extract, WPM2.5, and organic soluble extract, O-PM2.5) of PM2.5 on human lung epithelial cells (A549).
Methods
Samples were collected using a high-volume air sampler. Each sample was divided into two groups by its own types (water soluble extract, W-PM2.5, and organic soluble extract, O-PM2.5). In the present study, two types of PM2.5-induced cytotoxic and genotoxic effects and expression of toxicity-related genes were evaluated using human lung epithelial cells (A549). Also, the production of intracellular reactive oxygen species was measured to investigate the mechanism of cell death induced by PM2.5
Results
Both W-PM2.5 and O-PM2.5 exposures significantly reduced the viability of A549 cells in a dosedependent manner, and expression of 17 cell deathrelated genes were significantly regulated in the PM2.5 exposure group. Exposure of PM2.5 significantly induced the production of ROS. Further, data obtained from the Comet assay indicated that two extracts of PM2.5 caused DNA damage in A549 cells in a dose-dependent manner.
Conclusion
Our study suggests that ROS-mediated DNA damage may play a major role in PM2.5-induced cell death. This finding represents the basis for further studies addressing the pathophysiological mechanisms of PM2.5 exposure.
Similar content being viewed by others
References
Gualtieri, M. et al. Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol In Vitro 24, 29–39, doi:10.1016/j.tiv.2009.09.013 (2010).
Hetland, R. B. et al. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol In Vitro 18, 203–212 (2004).
Dreher, K. L. Particulate Matter Physicochemistry and Toxicology: In Search of Causality-A Critical Perspective. Inhal Toxicol 12(Suppl 3), 45–57, doi:10.1080/08 958378.2000.11463230 (2000).
G ualtieri, M. et al. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol Lett 209, 136–145, doi:10.1016/j.toxlet.2011.11.015 (2012).
Gutierrez-Castillo, M. E. et al. Effect of chemical composition on the induction of DNA damage by urban airborne particulate matter. Environ Mol Mutagen 47, 199–211, doi:10.1002/em.20186 (2006).
Perrone, M. G. et al. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere 78, 1368–1377, doi:10.1016/j.chemosphere.2009.12.071 (2010).
Schiliro, T., Alessandria, L., Degan, R., Traversi, D. & Gilli, G. Chemical characterisation and cytotoxic effects in A549 cells of urban-air PM10 collected in Torino, Italy. Environ Toxicol Pharmacol 29, 150–157, doi:10.1016/j.etap.2009.12.005 (2010).
Kouassi, K. S. et al. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Cote d'Ivoire. J Appl Toxicol 30, 310–320, doi:10.1002/jat.1496 (2010).
Campbell, A., Araujo, J. A., Li, H., Sioutas, C. & Kleinman, M. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol 9, 5099–5104 (2009).
Choi, Y. S. et al. Toxicity of low doses of ultrafine diesel exhaust particles on bovine brain microvessel endothelial cells. Mol Cell Toxicol 10, 245–250 (2014).
Alfaro-Moreno, E. et al. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ Health Perspect 110, 715–720 (2002).
Jeong, S. C., Shin, C. Y., Song, M. K., Cho, Y. & Ryu, J. C. Gene expression profiling of human alveolar epithelial cells (A549 cells) exposed to atmospheric particulate matter 2.5 (PM2.5) collected from Seoul, Korea. Mol Cell Toxicol 10, 361–368 (2014).
Li, N., Xia, T. & Nel, A. E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Bio Med 44, 1689–1699 (2008).
Soleimani, E., Moghadam, R. H. & Ranjbar, A. Occupational exposure to chemicals and oxidative toxic stress. Toxicol Environ Health Sci 7, 1–24 (2015).
Shin, C.-Y. et al. Extraction method for manipulation of water-and organic-soluble extracts of PM2.5 in Korean winter season and its chemical composition. Toxicol Environ Health Sci 5, 55–64 (2013).
Lai, S. C., Zou, S. C., Cao, J. J., Lee, S. C. & Ho, K. F. Characterizing ionic species in PM2.5 and PM10 in four Pearl River Delta cities, south China. J Environ Sci 19, 939–947 (2007).
Vasilakos, C. et al. Temporal determination of heavy metals in PM2.5 aerosols in a suburban site of Athens, Greece. J Atmos Chem 57, 1–17 (2007).
Voutsa, D. & Samara, C. Labile and bioaccessible fractions of heavy metals in the airborne particulate matter from urban and industrial areas. Atmos Environ 36, 3583–3590 (2002).
Colin, D. J. et al. The role of reactive oxygen species and subsequent DNA-damage response in the emergence of resistance towards resveratrol in colon cancer models. Cell Death Dis 5, e1533, doi:10.1038/cddis. 2014.486 (2014).
Wezel, A. et al. Deficiency of the TLR4 analogue RP105 aggravates vein graft disease by inducing a pro-inflammatory response. Sci Rep-Uk 6, 24248, doi:10.1038/srep24248 (2016).
Monn, C. & Becker, S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol Appl Pharmacol 155, 245–252, doi:10.1006/taap.1998.8591 (1999).
Suarez, A. E. & Ondov, J. M. Ambient aerosol concentrations of elements resolved by size and by source: Contributions of some cytokine-active metals from coal-and oil-fired power plants. Energ Fuel 16, 562–568 (2002).
Lee, J. Y., Kim, Y. P. & Kang, C. H. Characteristics of the ambient particulate PAHs at Seoul, a mega city of Northeast Asia in comparison with the characteristics of a background site. Atmos Res 99, 50–56 (2011).
Froehner, S., Maceno, M., Machado, K. S. & Grube, M. Health risk assessment of inhabitants exposed to PAHs particulate matter in air. J Environ Sci Health A Tox Hazard Subst Environ Eng 46, 817–823, doi:10.1080/10934529.2011.579843 (2011).
Shen, G. et al. Emission characteristics for polycyclic aromatic hydrocarbons from solid fuels burned in domestic stoves in rural China. Environ Sci Technol 47, 14485–14494, doi:10.1021/es403110b (2013).
Osornio-Vargas, A. R. et al. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect 111, 1289–1293 (2003).
Knaapen, A. M., Shi, T. M., Borm, P. J. A. & Schins, R. P. F. Soluble metals as well as the insoluble particle fraction are involved in cellular DNA damage induced by particulate matter. Mol Cell Biochem 234, 317–326 (2002).
Soberanes, S. et al. p53 mediates particulate matterinduced alveolar epithelial cell mitochondria-regulated apoptosis. Am J Respir Crit Care Med 174, 1229–1238, doi:10.1164/rccm.200602-203OC (2006).
Song, M. K. et al. Formation of a 3,4-diol-1,2-epoxide metabolite of benz[a]anthracene with cytotoxicity and genotoxicity in a human in vitro hepatocyte culture system. Environ Toxicol Pharmacol 33, 212–225, doi:10.1016/j.etap.2011.12.020 (2012).
Kauppinen, A., Suuronen, T., Ojala, J., Kaarniranta, K. & Salminen, A. Antagonistic crosstalk between NFkappa B and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25, 1939–1948 (2013).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappa B activation by TNFR family members. Nature 424, 793–796 (2003).
Han, M. K. et al. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2, 241–251, doi:10.1016/j.stem.2008.01.002 (2008).
Kaltschmidt, B. et al. The pro-or anti-apoptotic function of NF-κB is determined by the nature of the apoptotic stimulus. Eur J Biochem 267, 3828–3835 (2000).
Bala, S. & Tabaku, A. Chronic obstructive pulmonary disease in iron-steel and ferrochrome industry workers. Cent Eur J Public Health 18, 93–98 (2010).
Michael, S., Montag, M. & Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ Pollut 183, 19–29, doi:10.1016/j.envpol.2013. 01.026 (2013).
Cao, D., Bromberg, P. A. & Samet, J. M. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol 37, 232–239, doi:10.1165/rcmb.2006-0449OC (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, W., Jeong, SC., Shin, Cy. et al. A study of cytotoxicity and genotoxicity of particulate matter (PM2.5) in human lung epithelial cells (A549). Mol. Cell. Toxicol. 14, 163–172 (2018). https://doi.org/10.1007/s13273-018-0018-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-018-0018-0