Abstract
Plants are sessile organisms. They have to endure the environmental catastrophe throughout their life cycle. To survive in these hostile surroundings, plants posses an efficient and fine-tuned defense mechanism. This is a well established fact that various defense molecules viz. salicylic acid (SA), jasmonic acid, abscisic acid (ABA), ethylene (ET) and so on, are working in a synergistic as well as antagonistic fashion to establish and activate the effective defense mechanism. Interestingly, glutathione is gaining a gradual importance in this complex scenario. This multifunctional biomolecule exists in two forms. The reduced form, viz. GSH, is primarily present at millimolar concentrations in various plant tissues as compared to its oxidized form, glutathione disulfide, GSSG. Proteo-genomics analysis confirmed that GSH plays a vital role in plant resistance against biotic and abiotic stresses by stimulating various defense genes and proteins. In recent times, it has been reported that modulation of GSH contents transmits information through diverse signaling mechanisms. GSH also modulates various stresses and defense related genes by interacting with ABA and ET in response to abiotic stress conditions. However, there are still many unanswered questions about the intricate molecular mechanism of GSH’s contribution in plant defense. With these backgrounds, presently we primarily discussed the transcriptomic changes under stress conditions in Arabidopsis thaliana at altered GSH contents. Transcriptomic profiling of phytoalexin-deficient mutant (pad2.1), a GSH depleted A. thaliana mutant, in response to combined cold and osmotic stress treatment, was compared to that of A. thaliana ecotype Col-0, the wild type, with a view to identify the genes altered under changed GSH conditions to combat stress. It was evident from these datasets that the transcript level responses of pad2.1 to this treatment were massive. Again, analysis of combined cold and osmotic stress treated other mutants of Arabidopsis transcriptome was performed to elucidate the crosstalk between the ABA, ET and GSH. Results revealed the differential regulation of about 2313 and 4131 transcripts in A. thaliana mutants viz. ethylene insensitive (ein2) and ABA deficient 1(aba1.6) respectively. Together, present findings elucidate an active interplay of GSH with SA, ET, and ABA to combat environmental stress conditions in planta.



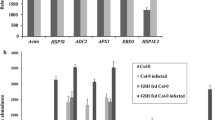
(Source: PLoS One. 2015;10: e0122690)

(Source: Sci Reports. 2016;15:36867)
Similar content being viewed by others
References
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. https://doi.org/10.1016/j.cell.2006.02.01.
Alexander D, Stinson J, Pear J, Glascock C, Ward E, Goodman RM, Ryals J. A new multigene family inducible by tobacco mosaic virus or salicylic acid in tobacco. Mol Plant-Microbe Interact. 1992;5:513–5.
Balabanova D, Remans T, Vessilev A, Cuypers A, Vangronsveld J. Possible involvement of glutathione S-transferases in imazamox detoxification in an imidazolinone-resistant sunflower hybrid. J Plant Physiol. 2018;221:62–5. https://doi.org/10.1016/j.jplph.2017.12.008.
Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A. Evidence for a direct link between glutathione biosynthesis and stress defence gene expression in Arabidopsis. Plant Cell. 2004;16:2448–62. https://doi.org/10.1105/tpc.104.022608.
Blanco F, Salinas P, Cecchini NM, Jordana X, Van Hummelen P, Alvarez ME, Holuigue L. Early genomic responses tosalicylic acid in Arabidopsis. Plant Mol Biol. 2009;70:79–102. https://doi.org/10.1007/s11103-009-9458-1.
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–93.
Bradley DJ, Kjellbom P, Lamb CJ. Elicitor and wound induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. https://doi.org/10.1016/0092-8674(92)90530-P.
Brown DM, Upcroft JA, Upcroft P. Cysteine is the major low-molecular weight thiol in Giardia duodenalis. Mol Biochem Parasitol. 1993;61:155–8. https://doi.org/10.1016/S0166-6851(96)02776-4.
Burow M, Zhang Z-Y, Ober JA, Lambrix VM, Wittstock U, Gershenzon J, Kliebenstein DJ. ESP and ESM1 mediateindol-3-acetonitrile production from indol-3-ylmethyl glucosinolatein Arabidopsis. Phytochemistry. 2008;69:663–71. https://doi.org/10.1016/j.phytochem.2007.08.027.
Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. https://doi.org/10.1016/S0092-8674(00)81858-9.
Cheng MC, Ko K, Chang WL, Kuo WC, Chen GH, Lin TP. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015;83:926–39. https://doi.org/10.1111/tpj.12940
Choudhury FK, Devireddy AR, Azad RK, Shulaev V, Mittler R. Rapid accumulation of glutathione during light stress in Arabidopsis. Plant Cell Physiol. 2018;59:1817–26. https://doi.org/10.1093/pcp/pcy101.
Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamyl cysteine synthase. Plant J. 1998;16(1):73–8. https://doi.org/10.1046/j.1365-313x.1998.00262.x.
Copley SD, Dhillon JK. Lateral gene transfer and parallel evolution in the history of glutathione biosynthesis genes. Genome Biol. 2002. https://doi.org/10.1186/gb-2002-3-5-research0025.
Datta R, Chattopadhyay S. Glutathione as a crucial modulator of phytohormone signalling during pathogen defence in plants. Proc Ind Natl Sci Acad. 2018. https://doi.org/10.16943/ptinsa/2018/49349.
Datta R, Kumar D, Sultana A, Hazra S, Bhattacharyya D, Chattopadhyay S. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiol. 2015;169:2963–81.
De Vos M, Zaanen WV, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CMJ. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006;142:352–63. https://doi.org/10.1104/pp.106.083907.
Dong X. SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–23. https://doi.org/10.1016/1369-5266(88)80053-0.
Dron M, Clouse SD, Dixon RA, Lawton MA, Lamb CJ. Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. PNAS. 1988;85:6738–42. https://doi.org/10.1073/pnas.85.18.6738.
Ellis JE, Yarlett N, Cole D, Humphreys MJ, Lloyd D. Antioxidant defenses in the microaerophlic protozoan Trichomontas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology. 1994;140:2489–94.
Fahey RC, Newton GL, Arrick B, Overdang-Bogart T, Aley SB. Entamoeba histolytica: a eukaryote without glutathione metabolism. Science. 1984;224:70–2.
Fatma M, Asgher M, Masood A, Khan NA. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot. 2014;107:55–63. https://doi.org/10.1016/j.envexpbot.2014.05.008.
Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 2011;155(1):2–18. https://doi.org/10.1104/pp.110.167569.
Ghanta S, Bhattacharyya D, Sinha R, Banerjee A, Chattopadhyay S. Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta. 2011;233:895–910. https://doi.org/10.1007/s00425-011-1349-4.
Ghanta S, Datta R, Bhattacharyya D, Sinha R, Kumar D, Hazra S, Mazumder AB, Chattopadhyay S. Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J Plant Physiol. 2014. https://doi.org/10.1016/j.jplph.2014.03.002.
Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. PNAS. 1994;91:8955–9. https://doi.org/10.1073/pnas.91.19.8955.
Gomez LD, Noctor G, Knight MR, Foyer CH. Regulation of calcium signaling and gene expression by glutathione. J Exp Bot. 2004;55:1851–9. https://doi.org/10.1093/jxb/erh202.
Grant MR, Jones JDG. Hormone (dis) harmony moulds plant health and disease. Science. 2009;324:750–2. https://doi.org/10.1126/science.1173771.
Grill D, Tausz M, Tausz MM, De Kok LJ. Significance of glutathione in plant adaptation to the environment, vol. 2nd, vol. Dordrecht: Kluwer; 2001.
Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–53. https://doi.org/10.1074/jbc.M312846200.
Hartmann TN, Fricker MD, Rennenberg H, Meyer AJ. Cell-specific measurement of cytosolic glutathione in poplar leaves. Plant Cell Environ. 2003;26:965–75. https://doi.org/10.1046/j.1365-3040.2003.01031.x.
Hell R, Bergmann L. γ-Glutamylcysteinesynthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–12.
Hell R, Bergmann L. Glutathione synthetase in tobacco suspension cultures: catalytic properties and localization. Physiol Planta. 1988;72:70–6. https://doi.org/10.1111/j.1399-3054.1988.tb06624.x.
Henmi K, Demura T, Tsuboi S, Fukuda H, Iwabuchi M, Ogawa K. Change in the redox state of glutathione regulates differentiation of tracheary elements in Zinnia cells and Arabidopsis roots. Plant Cell Physiol. 2005;46(11):1757–65. https://doi.org/10.1093/pcp/pci198.
Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, Araki R, Sakurai N, Suzuki H, Aoki K, Goda H, Nishizawa OI, Shibata D, Saito K. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc Natl Acad Sci. 2007;104:6478–83. https://doi.org/10.1073/pnas.0611629104.
Hiruma K, Fukunaga S, Bednarek P, Pislewska-Bednarek M, Watanabe S, Narusaka Y, Shirasu K, Takano Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc Natl Acad Sci. 2013;110:9589–94. https://doi.org/10.1073/pnas.1305745110.
Horibe T, Nagai H, Sakakibara K, Hagiwara Y, Kikuchi M. Ribostamycin inhibits the chaperone activity of protein disulfide isomerase. Biochem Biophys Res Comm. 2001;5:965–72. https://doi.org/10.1006/bbrc.2001.6105.
Huan C, Jiang L, An X, Kang R, Yu M, Ma R, Yu Z. Potential role of glutathione peroxidase gene family in peach fruit ripening under combined postharvest treatment with heat and 1-MCP. Postharvest Biol Technol. 2016;111:175–84. https://doi.org/10.1016/j.postharvbio.2015.08.016.
Islam S, Choudhury M, Majlish K, Islam T, Ghosh A. Comprehensive genome-wide analysis of Glutathione S-transferase gene family in potato (Solanum tuberosum L.) and their expression profiling in various anatomical tissues and perturbation conditions. Gene. 2018;639:149–62. https://doi.org/10.1016/j.gene.2017.10.007.
Janowiak BE, Griffith OW. Glutathione Synthesis in Streptococcus agalactiae. J Biol Chem. 2005;280:11829–39. https://doi.org/10.1074/jbc.M414326200.
Joshi NC, Meyer AJ, Bangash SAK, Zheng ZL, Leustek T. Arabidopsis γ-glutamylcyclotransferase affects glutathione content and root system architecture during sulfur starvation. New Phytol. 2018. https://doi.org/10.1111/nph.15466.
Kao CW, Bakshi M, Sherameti I, Dong S, Reichelt M, Oelmüller R, Yeh KW. A Chinese cabbage (Brassica campetris subsp. Chinensis) τ-type glutathione-S-transferase stimulates Arabidopsis development and primes against abiotic and biotic stress. Plant Mol Biol. 2016;92:643–59. https://doi.org/10.1007/s11103-016-0531-2.
Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, Trifa Y, Pontier D, Lam E, Silva H. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci. 2000;97(16):8849–55. https://doi.org/10.1073/pnas.97.16.8849.
Kovacs I, Durner J, Lindermayr C. Crosstalk between nitric oxide and glutathione is required for NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1(NPR1)-dependent defense signaling in Arabidopsis thaliana. New Phytol. 2015;208:860–72. https://doi.org/10.1111/nph.13502.
Kumar D, Chattopadhyay S. Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis. J Exp Bot. 2018;69:3729–43. https://doi.org/10.1093/jxb/ery166.
Kumar D, Datta R, Hazra S, Sultana A, Mukhopadhyay R, Chattopadhyay S. Transcriptomic profiling of Arabidopsis thaliana mutant pad2.1 in response to combined cold and osmotic stress. PLoS ONE. 2015;10:e0122690. https://doi.org/10.1371/journal.pone.0122690.
Kumar D, Hazra S, Datta R, Chattopadhyay S. Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA, ethylene and GSH against combined cold and osmotic stress. Sci Rep. 2016;15:36867. https://doi.org/10.1038/srep36867.
Kumar S, Trivedi PK. Glutathione S-Transferases: role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front Plant Sci. 2018;9:751. https://doi.org/10.3389/fpls.2018.00751.
Kumari N, Jain V, Talwar G. Salinity induced changes in ascorbic acid, hydrogen peroxide, lipid peroxidation and glutathione content in leaves of salt tolerant and salt-susceptible cultivars of cotton (Gossypium hirsutum L.). Res. Plant Biol. 2013;3:06–11.
Kunert KJ, Foyer CH. Thiol/disulphide exchanges in plants. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rausen W, editors. Sulphur Nutrition and Assimilation in Higher Plants. Regulatory, Agricultural and Environmental Aspects. The Hague: SPB Academic Publishers; 1993. p. 139–51.
Kunkel BN, Brooks DM. Cross-talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–31.
Kuźniak E, Kopczewski T, Koźniewska JC. Ascorbate-glutathione cycle and biotic stress tolerance in plants. In: Hossain MA, Munné-Bosch S, Burritt DJ, Diaz-Vivancos P, Fujita M, Lorence A, editors. Ascorbic Acid in Plant Growth, Development and Stress tolerance. Cham: Springer; 2017. p. 201–31.
Labade CP, Jadhav AR, Ahire M, Zinjarde SS, Tamhane VA. Role of induced glutathione-S-transferase from Helicoverpa armigera (Lepidoptera: noctuidae) HaGST-8 in detoxification of pesticides. Ecotoxicol Environ Saf. 2018;147:612–21. https://doi.org/10.1016/j.ecoenv.2017.09.028.
Lai Z, Vinod KM, Zheng Z, Fan B, Chen Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol. 2008;8:68. https://doi.org/10.1186/1471-2229-8-68.
Lawton KA, Potter SL, Uknes S, Ryals J. Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell. 1994;6:581–90.
Li H, Xu H, Graham DE, White RH. Glutathione synthatase homologs encode α-L-glutamate ligases for methanogenic coenzyme F420 and tetrahydrosarcinapterin biosyntheses. Proc Natl Acad Sci. 2003. https://doi.org/10.1073/pnas.1733391100.
Lin TH, Rao MY, Lu HW, Chiou CW, Lin ST, Chao HW, Zheng ZL, Cheng HC, Lee TM. A role for glutathione reductase and glutathione in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Physiol Plant. 2018;162:35–48. https://doi.org/10.1111/ppl.12622.
Matern S, Berghoefer TP, Gromes R, Kiesel RV, Rausch T. Imposed glutathione-mediated redox switch modulates the tobacco wound-induced protein kinase and salicylic acid-induced protein kinase activation state and impacts on defence against Pseudomonas syringae. J Exp Bot. 2015;66:1935–50. https://doi.org/10.1093/jxb/eru546.
Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–8.
Meyer AJ, Fricker MD. Control of demand-driven biosynthesis of glutathione in green Arabidopsis suspension culture cells. Plant Physiol. 2002;130:1927–37. https://doi.org/10.1104/pp.008243.
Meyer AJ, May MJ, Fricker M. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J. 2001;27:67–78. https://doi.org/10.1046/j.1365-313x.2001.01071.x.
Mhamdi A. Managing competing interests: partitioning S between glutathione and protein synthesis. Plant Physiol. 2018;177:867–8. https://doi.org/10.1104/pp.18.00661.
Moura JCMS, Bonine CAV, de Oliveira Fernandes Viana J, Dornelas MC, Mazzafera P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol. 2010;52:360–76. https://doi.org/10.1111/j.1744-7909.2010.00892.x.
Mukherjee AK, Carp MJ, Zuchman R, Ziv T, Horwitz BA, Gepstein S. Proteomics of the response of Arabidopsis thaliana to infection with Alternaria brassicicola. J Proteom. 2010;73:709–20. https://doi.org/10.1016/j.jprot.2009.10.005.
Noctor G, Foyer CH. ASCORBATE AND GLUTATHIONE: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol. 1998;49:249–79.
Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–66.
Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–82. https://doi.org/10.1105/tpc.7.2.173.
Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a gamma-glutamylcysteinesynthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007;49:159–72. https://doi.org/10.1111/j.1365-313X.2006.02938.x.
Passaia G, Pinheiro MM. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015;234:22–6. https://doi.org/10.1016/j.plantsci.2015.01.017.
Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–16. https://doi.org/10.1038/nchembio.164.
Rennenberg H. Molecular approaches to glutathione biosynthesis. In: Cram WJ, DeKok LJ, Stulen I, Brunold C, Renneberg H, editors. Sulphur metabolism in higher plants; molecular, ecophysiological and nutritional aspects. Leiden: Backhuys Publishers; 1997. p. 59–70.
Rüegsegger A, Brunold C. Localization of γ–glutamylcysteine synthetase and glutathione synthetase activity in maize seedling. Plant Physiol. 1993;101:380–90.
Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 2008;55:774–86. https://doi.org/10.1111/j.1365-313X.2008.03545.x.
Semida WM, Hemida KA, Rady MM. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol Environ Safety. 2018;154:171–9. https://doi.org/10.1016/j.ecoenv.2018.02.036.
Shepherd RW, Bass WT, Houtz RL, Wagner GJ. Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell 2005;17:1851–61. https://doi.org/10.1105/tpc.105.031559.
Shepherd RW, Wagner GJ. Phylloplane proteins: Emerging defenses at the aerial frontline? Trends Plant Sci. 2007;12:51–6.
Singla-Pareek SL, Reddy MK, Sopory SK. Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Nat Acad Sci. 2003;100:14672–7. https://doi.org/10.1073/pnas.2034667100.
Sinha R, Kumar D, Datta R, Hazra S, Bhattacharyya D, Mazumdar AB, Mukhopadhyay R, Sultana A, Chattopadhyay S. Integrated transcriptomic and proteomic analysis of Arabidopsis thaliana exposed to glutathione unravels its role in plant defense. Plant Cell Tissue Org Cult. 2014;120:975–88.
Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Natl Rev Immunol. 2012;12:89–100.
Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. Differential regulation of closely relatedR2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50:660–77. https://doi.org/10.1111/j.1365-313X.2007.03078.x.
Suttipanta N, Pattanaik S, Kulshrestha M, Patra B, Singh SK, Yuan L. The transcription factor CrWRKY1 positively regulates the terpenoidindole alkaloid biosynthesis in Catharanthusroseus. Plant Physiol. 2011;157:2081–93.
Teh CY, Mahmood M, Shaharuddin NA, Ho CL. In vitro rice shoot apices as simple model to study the effect of NaCL and the potential exogenous proline and glutathione in mitigating salinity stress. Plant Growth Regul. 2014;75(3):771–81. https://doi.org/10.1007/s10725-014-9980-2.
Tyagi S, Himani Sembi JK, Gene Upadhyay SK. architecture and expression analyses provide insights into the role of glutathione peroxidases (GPXs) in bread wheat (Triticum aestivum L.). J Plant Physiol. 2018;223:19–31. https://doi.org/10.1016/j.jplph.2018.02.006.
Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Montagu MV, Inzé D, May MJ, Sung XR. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–109.
Vlot AC, Klessig DF, Park SW. Systemic acquired resistance: the elusive signal(s). Curr Opin Plant Biol. 2008;11:436–42. https://doi.org/10.1016/j.pbi.2008.05.003.
Wingate VPM, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988;87:206–10.
Wu TM, Lin WR, Kao CH, Hong CY. Gene knockout of glutathione reductase 3 results in increased sensitivity to salt stress in rice. Plant Mol Biol. 2015;87:555–64. https://doi.org/10.1007/s11103-015-0290-5.
Xiang C, Werner BL, Christensen EM, Oliver DJ. The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001;126:564–74.
Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol. 1984;35:155–89.
Yang Z, Tian L, Latoszek-Green M, Brown D, Wu K. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol Biol. 2005;58:585–96.
Yoshida S, Tamaoki M, Ioki M, Ogawa D, Sato Y, Aono M, Kubo A, Saji S, Saji H, Satoh S, Nakajima N. Ethylene and salicylic acid control glutathione biosynthesis in ozone-exposed Arabidopsis thaliana. Physiol Plant. 2009;136:284–98. https://doi.org/10.1111/j.1399-3054.2009.01220.x.
Zhang Q, Zhu J, Ni Y, Cai Y, Zhang Z. Expression profiling of HbWRKY1, an ethephon-inducedWRKY gene in latex from Hevea brasiliensis in responding to wounding and drought. Trees. 2012;26:587–95.
Acknowledgements
The authors would like to acknowledge the help and support of the Director, CSIR-IICB. This work received financial assistance from the Science and Engineering Research Board, (SERB) and Council of Scientific and Industrial Research (CSIR), New Delhi. PB and AS would like to acknowledge the CSIR for their fellowships. Scientific Reports (Nature Publishing Group) and Plos One (Publication Library of Science) is acknowledged herewith to reuse the figures (Figs. 4, S2, S3, and 3) respectively. Authors would like express their gratitude for invitation to contribute in this special issue of the journal, The Nucleus.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to the memory of Profs. AK Sharma and Archana Sharma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boro, P., Sultana, A., Mandal, K. et al. Transcriptomic changes under stress conditions with special reference to glutathione contents. Nucleus 61, 241–252 (2018). https://doi.org/10.1007/s13237-018-0256-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-018-0256-5