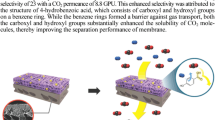
Abstract
We found that membranes consisting of poly(ethylene oxide) (PEO), AgBF4, and Al(NO3)3 exhibit relatively high permeance, i.e., 20 gel permeation unit (GPU) with a mixed-gas selectivity of 10 for propylene/propane separation. To enhance separation, high-molecular-weight PEO was utilized as polymer matrix. As a result, when 9.0×105 g/mol PEO was utilized, the PEO/AgBF4/Al(NO3)3 electrolyte membranes showed permeance of 32 GPU with a selectivity of 11, as well as demonstrating long-term stability. It was found that longer polymer chains could enable extensive segmental motions that enhance olefin diffusion, thereby improving gas performance. Furthermore, the more viscous aqueous solution of high-molecular-weight PEO enabled the formation of a thinner layer, thus improving the mix-gas permeance of the membrane, using the same conditions as low-molecular-weight PEOs. The coordination interactions of metal ions and ether moieties in the same electrolyte membrane were investigated by FT-IR and X-ray photoelectron spectroscopy.

Similar content being viewed by others
References
R. T. Yang and E. S. Kikkinides, AIChE J., 41, 509 (1995).
T. Ren, M. Patel, and K. Blok, Energy, 31, 425 (2006).
D. M. Ruthve and S. C. Reyes, Micropor. Mesopor. Mat., 104, 59 (2007).
P. Krokidas, M. Castier, and I. G. Economou, J. Phys. Chem. C, 121, 17999 (2017).
R. J. Verploegh, S. Nair, and D. S. Sholl, J. Am. Chem. Soc., 137, 15760 (2015).
M. J. Lee, M. R. A. Hamid, J. Lee, J. S. Kim, Y. M. Lee, and H.-K. Jeong, J. Membrane Sci., 559, 28 (2018).
Z. R. Herm, E. D. Bloch, and J. R. Long, Chem. Mater., 26, 323 (2014).
J. Yu, C. Wang, L. Xiang, Y. Xu, and Y. Pan, Chem. Eng. Sci., 179, 1 (2018).
C. Gucuyener, V. D. Bergh, J. Gasco, and F. Kapteijn, J. Am. Chem. Soc., 132, 17704 (2010).
B. Li, H. Wang, and B. Chen, Chem. Asian J., 9, 1474 (2014).
B. Li, H. M. Wen, W. Zhou, and B. Chen, J. Phys. Chem. Lett., 5, 3468 (2014).
J. H. Lee, H. T. Kwon, S. Bae, J. Kim, and J. H. Kim, Sep. Purif. Technol., 207, 427 (2018).
R. L. Grantom, and D. J. Royer, Ethylene, Ullmann’s Encyclopedia of Industrial Chemistry, VCH, New York, 5th ed., 1987.
K. Wang and E. I. Stiefel, Science, 291, 106 (2001).
K. A. Smith, J. H. Meldon, and C. K. Colton, AIChE J., 19, 102 (1973).
W. S. Ho and D. C. Dalrymple, J. Membrane Sci., 91, 13 (1994).
M. Azhin, T. Kaghazchi, and M. Rahmani, J. Ind. Eng. Chem., 14, 622 (2008).
S. U. Hong, J. H. Jin, J. Won, and Y. S. Kang, Adv. Mater., 12, 968 (2000).
C. Staudt-Bickel and W. J. Koros, J. Membrane Sci., 170, 205 (2000).
I. G. Giannakopoulos and V. Nikolakis, Ind. Eng. Chem. Res., 44, 226 (2004).
M. L. Chng, Y. Xiao, T.-S. Chung, M. Toriida, and S. Tamai, Carbon, 47, 1857 (2009).
F. Y. Li, Y. Li, T. S. Chung, and S. Kawi, J. Membrane Sci., 356, 14 (2010).
Z. Qiao, Z. Wang, C. Zhang, S. Yuan, Y. Zhu, J. Wang, and S. Wang, AIChE J., 59, 215 (2013).
I. Pinnau and L. G. Toy, J. Membrane Sci., 184, 39 (2001).
I. Pinnau, L. G. Toy, and C. Casillas, US Patent 5,670,051 (23 Sep. 1997).
J. H. Ryu, H. Lee, Y. J Kim, Y. S. Kang, and H. S. Kim, Chem. Eur. J., 7, 1525 (2001).
S. W. Kang, J. H. Kim, J. Won, and Y. S. Kang, J. Membrane Sci., 445, 156 (2013).
D. Song, Y. S. Kang, and S. W. Kang, J. Membrane Sci., 474, 273 (2015).
Y. Li and Y. Hu, RSC Adv., 4, 51022 (2014).
K. Nakajima, S. Nagaoka, and H. Kawakami, Polym. Adv. Technol., 14, 433 (2003).
A. Halim, Q. Fu, Q. Yong, P. A. Gurr, S. E. Kentish, and G. G. Qiao, J. Mater. Chem. A, 2, 4999 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: This work was supported by the Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant (20122010100040), funded by the Ministry of Trade, Industry, and Energy of the Korean government. This work was also supported by the Basic Science Research Program (2017R1D1A1B03032583) through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning. The authors also appreciate Daeun Song’s technical support.
Rights and permissions
About this article
Cite this article
Chae, I.S., Kang, S.W. Enhanced Separation Performance of Stabilized Olefin Transport Membranes with High-Molecular-Weight Poly(ethylene oxide). Macromol. Res. 27, 511–514 (2019). https://doi.org/10.1007/s13233-019-7066-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-019-7066-8