Abstract
Background/Purpose
Misoprostol is beneficial in preventing postpartum haemorrhage (PPH). However, there is no consensus yet as to which route will give the balance of efficacy, safety and patient preference, especially at the recommended dose of 600 mcg. This study compared the efficacy and adverse effects of rectal and sublingual misoprostol for the prevention of PPH.
Methods
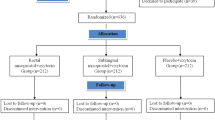
In a prospective fashion, consenting eligible parturients were randomised into two groups to receive either 600 mcg of misoprostol rectally or sublingually after vaginal delivery. All study participants were followed up till 24 h postpartum. Primary outcomes were blood loss of 500 ml or greater and at least 10% change in peripartum haematocrit levels.
Results
Seven (6.7%) and 16 (15.7%) of the sublingual and rectal routes, respectively, had PPH. However, the odds of having PPH after rectal misoprostol were at least twice the odds after the sublingual route (p = 0.041). Also, the mean blood loss after the first, fourth and 24th hour postpartum were significantly higher after rectal administration. Although significantly more patients had shivering and pyrexia after sublingual misoprostol, it was acceptable to more participants than the rectal route.
Conclusion
At the recommended dose, sublingually administered misoprostol (‘the sweet of life’) is associated with a lower incidence of PPH than the rectal route. Despite its higher incidence of shivering and pyrexia, it was accepted by more women than rectally administered misoprostol.
ClinicalTrials.gov identifier PACTR201911500348367.

Similar content being viewed by others
Data Availability
The data can be obtained from the trials registry, or from the authors after due permission from the institution.
References
United Nations. Transforming our world: The 2030 agenda for sustainable development. https://www.unfpa.org/resources/transforming-our-world-2030-agenda-sustainable-development. Accessed 5 Jan 2020.
Leduc D, Senikas V, Lalonde AB. Clinical practice obstetrics committee. active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31(10):980–93.
Oyelese Y, Ananth CV. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol. 2010;53(1):147–56.
Evensen A, Anderson JM, Fontaine P. Postpartum hemorrhage: prevention and treatment. Am Fam Physician. 2017;95(7):442–9.
World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548502/en/. Accessed 5 Jan 2020.
Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev. 2013;10:CD001808.
Ejembi C, Shittu O, Moran M, et al. Community-level distribution of misoprostol to prevent postpartum hemorrhage at home births in Northern Nigeria. Afr J Reprod Health. 2014;18(2):166–75.
Obiechina NJA, Eleje GU, Ezebialu IU, et al. Emergency peripartum hysterectomy in Nnewi, Nigeria: a 10-year review. Niger J Clin Pract. 2012;15(2):168–71.
Akintayo AA, Olagbuji BN, Aderoba AK, et al. Emergency peripartum hysterectomy: a multicenter study of incidence, indications and outcomes in Southwestern Nigeria. Matern Child Health J. 2016;20(6):1230–6.
Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;3:CD007412.
Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–33.
Mobeen N, Durocher J, Zuberi N, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG. 2011;118(3):353–61.
Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet. 2007;99(Suppl 2):S160–7.
Alwani M, Singh S, Thakur R, et al. A randomised study comparing rectally administered misoprostol after spinal anesthesia versus intramuscular oxytocin for prevention of postpartum hemorrhage in caesarean section. Int J Reprod Contracept Obstet Gynecol. 2014;3(3):512–5.
Hofmeyr GJ, Gülmezoglu AM, Novikova N, et al. Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects. Bull World Health Organ. 2009;87(9):666–77.
Musa AO, Ijaiya MA, Saidu R, et al. Double-blind randomised controlled trial comparing misoprostol and oxytocin for management of the third stage of labour in a Nigerian hospital. Int J Gynecol Obstet. 2015;129(3):227–30.
Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012;8:CD000494.
Ugwu IA, Oluwasola TA, Enabor OO, et al. Randomised controlled trial comparing 200 μg and 400 μg sublingual misoprostol for prevention of primary postpartum hemorrhage. Int J Gynaecol Obstet. 2016;133(2):173–7.
Elati A, Elmahaishi MS, Elmahaishi MO, et al. The effect of misoprostol on postpartum contractions: a randomised comparison of three sublingual doses. BJOG. 2011;118(4):466–73.
National Population Commission (NPC) (2010) 2006 population and housing census priority tables, vol 4. National Population Commission (NPC), Abuja.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Atukunda EC, Siedner MJ, Obua C, et al. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial. PLoS Med. 2014;11(11):e1001752.
Mavis N. Schorn CNM. J Midwifery Womens Health: Measurement of blood loss: review of the literature; 2010. p. 20–7.
Frass KA. Postpartum hemorrhage is related to the hemoglobin levels at labour: an observational study. Alex J Med. 2015;51(4):333–7.
Nagasree MGS, Smitha A. Misoprostol versus oxytocin in prevention of postpartum hemorrhage. Int J Basic Appl Med Sci. 2015;5(1):180–5.
Samal R, Coumary SA, John LJ, Ghose S. Comparative study of oral, rectal misoprostol with intravenous methylergometrine in active management of third stage of labour. J Pharm Biomed Sci. 2014;4(9):828–33.
Al-Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum haemorrhage. Saudi Med J. 2009;30(7):912–6.
Udayan MA, Hareshbhai PP. A study on efficacy and safety of the drug misoprostol 600 mcg for prevention of postpartum hemorrhage by different routes of administration in routine management of 3rd stage of labor: a randomised placebo controlled double blind study. Gujarat Med J. 2014;69(2):58–64.
Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in the active management of the third stage of labor. Obstet Gynecol Surv. 2009;107(2):130–4.
Nasreen HE, Nahar S, Mamun MA, et al. Oral misoprostol for preventing postpartum haemorrhage in home births in rural Bangladesh: how effective is it? Glob Health Action. 2011;4:1.
Fawzy AEA, Swelem M, Abdelrehim AI, et al. Active management of third stage of labor by intravenous ergometrine and rectal versus sublingual misoprostol (a double-center study). Alex J Med. 2012;48(4):381–5.
Vagge DS, Mamatha KR, Shivamurthy G, et al. A comparative study to assess the efficacy and tolerability of per rectal misoprostol and intravenous oxytocin in prevention of primary postpartum haemorrhage. J Chem Pharm Res. 2014;6(3):1134–40.
Harriott J, Christie L, Wynter S, et al. A randomised comparison of rectal misoprostol with syntometrine on blood loss in the third stage of labour. West Indian Med J. 2009;58(3):201–6.
Adanikin A, Orji EO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage after caesarean section. Nepal J Obstet Gynaecol. 2016;8(16):34–7.
Zhao Z, Yang WZ, Gao C, et al. A hypothalamic circuit that controls body temperature. PNAS. 2017;114(8):2042–7.
Acknowledgements
The authors wish to acknowledge the contributions of the staff of the labour ward, the resident doctors and consultants in the Department to the success of the study.
Funding
The study was entirely self-funded.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by BTA and JOA. The first draft of the manuscript was written by JOA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interests.
Ethical Statement
The study was done in accordance with the 1964 Declaration of Helsinki. All recruited patients were adequately counselled and their written informed consent obtained. They were at liberty to decline participation without any negative impact on the quality of care they will receive in the hospital. The institution’s Ethics and Research Committee gave approval for the study, which was designed and reported in line with the CONSORT Statement 2010.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Awoleke is an Associate Professor in the Department of Obstetrics and Gynaecology, College of Medicine, Ekiti State University, Ado-Ekiti, Nigeria. Benedict Tolulope Adeyanju: MBChB in the Department of Obstetrics and Gynaecology, Ekiti State University Teaching Hospital, Ado – Ekiti, Nigeria, Adebayo Adeniyi: MBBS, FWACS, FMCOG in the Department of Obstetrics and Gynaecology, Federal Teaching Hospital, Ido – Ekiti, Nigeria, Olusola Peter Aduloju: MBBS, MPH, FWACS in the Department of Obstetrics and Gynaecology, Ekiti State University Teaching Hospital, Ado – Ekiti, Nigeria and Department of Obstetrics and Gynaecology, Ekiti State University Teaching Hospital, Ado – Ekiti, Nigeria, Babatunde Ajayi Olofinbiyi: MBBS, FWACS in the Department of Obstetrics and Gynaecology, Ekiti State University Teaching Hospital, Ado – Ekiti, Nigeria and Department of Obstetrics and Gynaecology, Ekiti State University Teaching Hospital, Ado – Ekiti, Nigeria.
Rights and permissions
About this article
Cite this article
Awoleke, J.O., Adeyanju, B.T., Adeniyi, A. et al. Randomised Controlled Trial of Sublingual and Rectal Misoprostol in the Prevention of Primary Postpartum Haemorrhage in a Resource-Limited Community. J Obstet Gynecol India 70, 462–470 (2020). https://doi.org/10.1007/s13224-020-01338-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-020-01338-0