Abstract
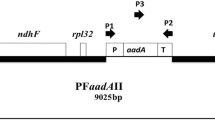
Chloroplast transformation vectors require an expression cassette flanked by homologous plastid sequences to drive plastome recombination. The rrn16-rrn23 plastome region was selected and using this region, a new species-specific plastid transformation vector CuIA was developed with pKS+II as a backbone by inserting the rrn16-trnI and trnA-rrn23 sequences from Cucumis sativus L. An independent expression cassette with aadA gene encoding aminoglycoside 3′-adenylyltransferase with psbA controlling elements is added into the trnI-trnA intergenic region that confers resistance to spectinomycin. An efficient plastid transformation in bitter melon (Momordica charantia L.) was achieved by bombardment of petiole segments. The frequency of transplastomic plants yielded using standardized biolistic parameters with CuIA vector was two per 15 bombarded plates, each containing 20 petiole explants. Integration of aadA gene was verified by PCR analysis in transplastomes. Transplastomic technology developed may be a novel approach for high level expression of pharmaceutical traits.






Similar content being viewed by others
References
Bock R (2014) Engineering plastid genomes: methods, tools and applications in basic research and biotechnology. Annu Rev Plant Biol 66:211–241
Carrer H, Hockenberry TN, Svab Z, Maliga P (1993) Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet 241:49–56
Cheng L, Li HP, Qu B, Huang T, Tu JX, Fu TD, Liao YC (2010) Chloroplast transformation of rapeseed (Brassica napus L.) by particle bombardment of cotyledons. Plant Cell Rep 29:371–381
Clarke JL, Daniell H (2011) Plastid biotechnology for crop production: present status and future perspectives. Plant Mol Biol 76:211–220
Cosa BD, Moar W, Lee SB, Miller M, Daniell H (2001) Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol 19:71–74
Daniell H, Lee SB, Grevich J, Saski C, Quesada-Vargas T, Guda C, Tomkins J, Jansen RK (2006) Complete chloroplast genome sequences of Solanum bulbocastanum, Solanum lycopersicum and comparative analyses with other Solanaceae genomes. Theor Appl Genet 112:1503–1518
DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H (2001) Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol 127:852–862
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull 19:11–15
Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker for site-directed transformation of chlamydomonas. Nucl Acids Res 19:4083–4089
Grover JK, Yadav SP (2004) Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol 93:123–132
Hou BK, Zhou YH, Wan LH, Zhang ZL, Shen GF, Chen ZH, Hu ZM (2003) Plastid transformation in oilseed rape. Transgenic Res 12:111–114
Iamtham S, Day A (2000) Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat Biotechnol 18:1172–1176
Kim JS, Jung JD, Lee JA, Park HW, Oh KH, Jeong WJ, Choi DW, Liu JR, Cho KY (2006) Complete sequence and organization of the cucumber (Cucumis sativus L. cv. Baekmibaekdadagi) chloroplast genome. Plant Cell Rep 25:334–340
Kanamoto H, Yamashita A, Asao H, Okumura S, Takase H, Hattori M, Yokota A, Tomizawa KI (2006) Efficient and stable transformation of Lactuca sativa L. cv. cisco (lettuce) plastids. Transgenic Res 15:205–217
Kavanagh TA, Thanh ND, Lao NT, McGrath N, Peter SO, Horváth EM, Dix PJ, Medgyesy P (1999) Homeologous plastid DNA transformation in tobacco is mediated by multiple recombination events. Genetics 152:1111–1122
Khan MS, Maliga P (1999) Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat Biotechnol 17:910–915
Lee SM, Kang K, Chung H, Yoo SH, Xu XM, Lee SB, Cheong JJ, Daniell H, Kim M (2006) Plastid transformation in the monocotyledonous cereal crop, rice (Oryza sativa) and transmission of transgenes to their progeny. Mol Cells 21:401–410
López-Ochoa LA, Apolinar-Hernández MM, Peña-Ramírez YJ (2015) Characterization of chloroplast region rrn16-rrn23S from the tropical timber tree Cedrela odorata L. and de novo construction of a transplastomic expression vector suitable for Meliaceae trees and other economically important crops. Genet Mol Res 14:1469–1478
Lugo SK, Kunnimalaiyaan M, Singh NK, Nielsen BL (2004) Required sequence elements for chloroplast DNA replication activity in vitro and in electroporated chloroplasts. Plant Sci 166(1):151–161
Maliga P (2004) Plastid transformation in higher plants. Annu Rev Plant Biol 55:289–313
Maliga P, Bock R (2011) Plastid biotechnology: food, fuel and medicine for the 21st century. Plant Physiol 155:1501–1510
Muralikrishna N, Srinivas K, BharathKumar K, Sadanandam A (2016) Stable plastid transformation in Scoparia dulcis L. Physiol Mol Biol Plants 22:575–581
Muralikrishna N, Raghu E, Srinivas K, Bharathkumar K, Yashodhara V, Sadanandam A (2018) Efficient genetic transformation of Momordica charantia L. by microprojectile bombardment. 3. Biotech 8:1–8
Rao AV, Krishna NM, Raghu E, Sadanandam A (2013) Genetic engineering of fruit rot disease resistance in Capsicum annuum L. using defensin gene (TvD1) through stable plastid transformation. In Vitro Cell Dev Biol. https://doi.org/10.1007/s11626-013-9614-4
Ruf S, Hermann M, Berger IJ, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol 19:870–875
Ruhlman T, Verma D, Samson N, Daniell H (2010) The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol 152:2088–2104
Serino G, Maliga P (1997) A negative selection scheme based on the expression of cytosine deaminase in plastids. Plant J 12:697–701
Sidorov VA, Kasten D, Pang SZ, Hajdukiewicz PTJ, Staub JM, Nehra NS (1999) Stable plastid transformation in potato: use of green fluorescent protein as a plastid marker. Plant J 19:209–216
Sikdar SR, Serino G, Chaudhuri S, Maliga P (1998) Plastid transformation in Arabidopsis thaliana. Plant Cell Rep 18:20–24
Sikdar B, Shafiullah M, Chowdhury AR, Sharmin N, Naha S, Joarder OI (2005) Agrobacterium-mediated GUS expression in bitter gourd (Momordica charantia L.). Biotechnology 4:149–152
Skarjinskaia M, Svab Z, Maliga P (2003) Plastid transformation in Lesquerella Fendleri, an oilseed brassicacea. Transgenic Res 12:115–122
Staub JM, Maliga P (1995) Expression of a chimeric uidA gene indicates that polycistronic mRNAs are efficiently translated in tobacco plastids. Plant J 7:845–848
Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90:913–917
Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87:8526–8530
Thiruvengadam M, Praveen N, Chung IM (2012) An efficient Agrobacterium tumefaciens -mediated genetic transformation of bitter melon (Momordica charantia L.). AJCS 6:1094–1100
Verma D, Daniell H (2007) Chloroplast vector systems for biotechnology applications. Plant Physiol 145:1129–1143
Wang HH, Yin WB, Hu ZM (2009) Advances in chloroplast engineering. J Genet Genomics 36:387–398
Yashodhara V, Muralikrishna N, Raghu E, Bharathkumar K, Rathnaprabha D, Sadanandam A (2016) In vitro plant regeneration from petiole explants and assessment of genetic fidelity using ISSR markers in Momordica charantia L. PCBMB 17:49–56
Ye GN, Hajdukiewicz P, Broyles D, Rodriguez D, Xu CW, Nehra N, Staub JM (2001) Plastid-expressed 5-enolpyruvylshikimate 3phosphate synthase genes provide high level glyphosate tolerance in tobacco. Plant J 25:261–270
Acknowledgements
MK would like to acknowledge the financial assistance provided by the Department of Science and Technology -INSPIRE (DST/INSPIRE Fellowship/2011/426), New Delhi. AS is grateful to UGC for BSR-Faculty Fellowship. The authors are also thankful to UGC, New Delhi for financial support under SAP-DRS phase-II to the Department of Biotechnology, Kakatiya University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Narra, M., Kota, S., Velivela, Y. et al. Construction of chloroplast transformation vector and its functional evaluation in Momordica charantia L.. 3 Biotech 8, 140 (2018). https://doi.org/10.1007/s13205-018-1160-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1160-z