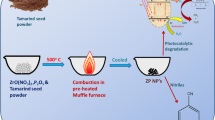
Abstract
The method of zinc (II) oxide synthesis by precipitation of zinc (II) acetate precursor has been developed. With the method of scanning electron microscopy, it was found that ZnO particles have a size of less than 100 nm. It contains a pure phase of zinc (II) oxide of the hexagonal syngony of the wurtzite type, which was proved by the X-ray diffraction method. The absorption spectrum of suspension of the obtained zinc (II) oxide powder was obtained and the optical bandgap was calculated by the Kubelka–Munk formula. The synthesized zinc (II) oxide is a well-crystallized solid material with semiconductor properties and a clear bandgap, starting at 3.1 eV. The resulting zinc (II) oxide has a BET surface area of 22 m2/g, as a result of which, its total porosity and micropore volume are small: 0.13 cm3/g and 0.06 cm3/g, respectively. It confirms that the obtained ZnO is a well-crystallized, non-porous material. The photocatalytic activity of the synthesized zinc (II) oxide is very high towards the dyes of both, anionic and cationic natures. The experimentally achieved values of the photocatalytic decomposition degree of the studied dyes indicate to an ambiguous influence of the model photocatalytic process organization on the efficiency of ZnO.








Similar content being viewed by others
References
Benhebal H, Chaib M, Salomon T et al (2013) Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by sol-gel process. Alex Eng J 52:517–523. https://doi.org/10.1016/j.aej.2013.04.005
Chong M, Jin B, Chow C et al (2010) Recent developments in photocatalytic water treatment technology. a review. Water Res 44:2997–3027. https://doi.org/10.1016/j.watres.2010.02.039
Djurišić A, Chen X, Leung Y (2012) Recent progress in hydrothermal synthesis of zinc oxide nanomaterials. Recent Pat Nanotechnol 6:124–134. https://doi.org/10.2174/187221012800270180
Dontsova T, Kutuzova A, Bila K et al (2020) Enhanced photocatalytic activity of TiO2/SnO2 binary nanocomposites. J Nanomat. https://doi.org/10.1155/2020/8349480
Guckan V, Altunal V, Ozdemir A et al (2020) Calcination effects on europium doped zinc oxide as a luminescent material synthesized via sol-gel and precipitation methods. J Alloys Compd 823:153878. https://doi.org/10.1016/j.jallcom.2020.153878
Hutsul Kh, Ivanenko I (2020) Struktura i vlastyvosti tsynku (II) oksydu. Ohliad Ekolohichni Nauky 2(29):140–144
International Organization for Standardization (ISO) (2010) Rubber compounding ingredients—zinc oxide—test methods: ISO 9298:1995. ISO, Geneva
Ivanenko I, Dontsova N, Astrelin I, Trots V (2016) Low-temperature synthesis, structure-sorption characterisics and photocatalytic activity of TiO2 nanostructures. J Wat Chem Technol 38:14–20. https://doi.org/10.3103/S1063455X16010033
Kukh A, Ivanenko I, Astrelin I (2019) TiO2 and its composites as effective photocatalyst for glucose degradation processes. Appl Nanosci 9:677–682. https://doi.org/10.1007/s13204-018-0691-2
Ludi B, Niederberger M (2013) Zinc oxide nanoparticles: chemical mechanism and classical and non-classical crystallization. Dalton Trans. https://doi.org/10.1039/c3dt50610j
Nikoobakht B, Wang X, Herzing A, Shi J (2013) Scable synthesis and device integration of self-registered one-dimensional zinc oxide nanostructures and related materials. Chem Soc Rev 42:342–365. https://doi.org/10.1039/C2CS35164A
Özgür Ü, Alivov Y, Liu C et al (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98(4):041301–041301103. https://doi.org/10.1063/1.1992666
Saboor A, Shah S, Hussain H (2019) Band gap tuning and applications of ZnO nanorods in hybrid solar cell: Ag-doped verses Nd-doped ZnO nanorods. Mater Sci Semicond Proc 93:215–225. https://doi.org/10.1016/j.mssp.2019.01.009
Stanković A, Veselinović L, Skapin S et al (2011) Controlled mechanochemically assisted synthesis of ZnO nanopowders in the presence of oxalic acid. J Mater Sci 46:3716–3724. https://doi.org/10.1007/s10853-011-5273-6
Xu T, Ji P, He M, Li J (2012) Growth and structure of pure ZnO micro/nanocombs. J Nanomater. https://doi.org/10.1155/2012/797935
Yue S, Yan Z, Shi Y, Ran G (2013) Synthesis of zinc oxide nanotubes within ultrathin anodic aluminum oxide membrane by sol-gel method. Mater Let 98:246–249. https://doi.org/10.1016/j.matlet.2013.02.037
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivanenko, I., Hutsul, K. & Krymets, G. The precipitation synthesis of zinc (II) oxide for photocatalytic degradation of anionic and cationic dyes. Appl Nanosci 12, 755–759 (2022). https://doi.org/10.1007/s13204-021-01694-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01694-x