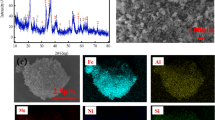
Abstract
The influence of various factors on the formation of nickel ferrite by the glow discharge effect has been studied. The ferritization process in the system FeSO4–NiSO4–NaOH–H2O has been studied by the methods of potentiometric titration, measurement of electrical conductivity, residual concentrations and apparent sediment volume. It has been established that the process proceeds in a multistage fashion at pH 11–12 with the formation of polyhydroxo complexes, an intermediate compound and the ferrite formation by its oxidation with active radicals.






Similar content being viewed by others
References
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42(5):053001
Chao X, Longjun X, Yongjun Y, Xiangyang L, Shuyun W (2015) Influence of pH on properties of Mn–Zn ferrites synthesized from low-grade manganese ore. Chin J Geochem 34(2):219–223
Costa RC, Lelis MFF, Oliveira LCA, Fabris JD, Ardisson JD, Rios RRVA, Lago RM (2006) Novel active heterogeneous Fenton system based on Fe3–xMxO4 (Fe Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions. J Hazard Mater 129(1–3):171–178
Dar MA, Shah J, Siddiqui WA, Kotnala RK (2014) Study of structure and magnetic properties of Ni–Zn ferrite nano-particles synthesized via co-precipitation and reverse micro-emulsion technique. Appl Nanosci 4(6):675–682
Diodati S, Pandolfo L, Caneschi A, Gialanella S, Gross S (2014) Green and low temperature synthesis of nanocrystalline transition metal ferrites by simple wet chemistry routes. Nano Res 7(7):1027–1042
Frolova LA, Derhachov MP (2017) The effect of contact non-equilibrium plasma on structural and magnetic properties of MnXFe3−XO4 Spinels. Nanoscale Res Lett 12(1):505
Frolova LA, Pivovarov AA (2015) Investigation of conditions for ultrasound-assisted preparation of nickel ferrite. High Energ Chem 49(1):10–15
Frolova LA, Pivovarov AA, Baskevich AS, Kushnerev AI (2014) Structure and properties of nickel ferrites produced by glow discharge in the Fe2+–Ni2+–SO4 2−–OH− system. Russ J Appl Chem 87(8):1054–1059
Galindo R, Menendez N, Crespo P, Velasco V, Bomati-Miguel O, Díaz-Fernández D, Herrasti P (2014) Comparison of different methodologies for obtaining nickel nanoferrites. J Magn Magn Mater 361:118–125
Hong D, Yamada Y, Nagatomi T, Takai Y, Fukuzumi S (2012) Catalysis of nickel ferrite for photocatalytic water oxidation using [Ru (bpy) 3]2+ and S2O8 2−. J Am Chem Soc 134(48):19572–19575
Hyeon T (2003) Chemical synthesis of magnetic nanoparticles. Chem Comm 8:927–934
Kamalvand P, Kumar Pandey G, Kumar Meshram M, Mallahzadeh A (2015) A single sided dual-antenna structure for UHF RFID tag applications. Int J Rf Microw C E 25(7):619–628
Klas S, Dubowski Y, Pritosiwi G, Gerth J, Calmano W, Lahav O (2011) Extent and mechanism of metal ion incorporation into precipitated ferrites. J Colloid Interface Sci 358(1):129–135
Krehula S, Popović S, Musić S (2002) Synthesis of acicular α-FeOOH particles at a very high pH. Mater Lett 54(2):108–113
Kurian M, Nair DS (2016) Effect of preparation conditions on nickel zinc ferrite nanoparticles: a comparison between sol–gel auto combustion and co-precipitation methods. J Saudi Chem Soc 20:517–522
Kuz’micheva LA, Titova YV, Maksimov AI, Vashurin AS, Pukhovskaya SG (2013) Effect of the cathode material on the accumulation of hydrogen peroxide in a plasma-solution system. Surf Eng Appl Electrochem 49(6):485–487
Kuz’michyova LA, Titova YV, Maksimov AI (2011) Yields of hydroxyl radicals and hydrogen peroxide in a glow discharge system with a liquid cathode. Surf Eng Appl Electrochem 47(6):517–519
Maaz K, Karim S, Mumtaz A, Hasanain SK, Liu J, Duan JL (2009) Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J Magn Magn Mater 321(12):1838–1842
Milichko VA, Nechaev AI, Valtsifer VA, Strelnikov VN, Kulchin YN, Dzyuba VP (2013) Photo-induced electric polarizability of Fe3O4 nanoparticles in weak optical fields. Nanoscale Res Lett 8(1):317–324
Morgan B, Lahav O (2007) The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution–basic principles and a simple heuristic description. Chemosphere 68(11):2080–2084
Nejati K, Zabihi R (2012) Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem Cent J 6(1):23
Pardavi-Horvath M (2000) Microwave applications of soft ferrites. J Magn Magn Mater 215:171–183
Peelamedu R, Grimes C, Agrawal D, Roy R, Yadoji P (2003) Ultralow dielectric constant nickel–zinc ferrites using microwave sintering. J Mater Res 18(10):2292–2295
Rawat J, Rana S, Srivastava R, Misra RDK (2007) Antimicrobial activity of composite nanoparticles consisting of titania photocatalytic shell and nickel ferrite magnetic core. Mater Sci Eng, C 27(3):540–545
Reddy CG, Manorama SV, Rao VJ (2000) Preparation and characterization of ferrites as gas sensor materials. J Mater Sci Lett 19(9):775–778
Rybkin VV, Shutov DA (2017) Atmospheric-pressure electric discharge as an instrument of chemical activation of water solutions. Plasma Phys Rep 43(11):1089–1113
Sinfrônio FSM, Santana PYC, Coelho SFN, Silva FC, de Menezes AS, Sharma SK (2017) Magnetic and structural properties of cobalt-and zinc-substituted nickel ferrite synthesized by microwave-assisted hydrothermal method. J Electron Mater 46(2):1145–1154
Tolchev AV, Kleschov DG, Bagautdinova RR, Pervushin VY (2002) Temperature and pH effect on composition of a precipitate formed in FeSO4–H2O–H+/OH−–H2O2 system. Mater Sci Technol phys 74(3):336–339
Tyagi AK, Ahlawat DS (2017) Influence of pH variation on structural and magnetic properties of Ni-Zn ferrite nanoparticles synthesized by auto combustion method. Orient J Chem 33(1):296–303
Uskoković V, Drofenik M (2005) A mechanism for the formation of nanostructured NiZn ferrites via a microemulsion-assisted precipitation method. Colloids Surf A Physicochem Eng Aspects 266(1-3):168–174
Vanetsev AS, Ivanov VK, Tret’yakov YD (2002) Microwave synthesis of lithium, copper, cobalt, and nickel ferrites. Dokl Chem 387(4–6):332–334
Vestal CR, Zhang ZJ (2003) Synthesis and magnetic characterization of Mn and Co spinel ferrite-silica nanoparticles with tunable magnetic core. Nano Lett 3(12):1739–1743
Wu KH, Yu CH, Chang YC, Horng DN (2004) Effect of pH on the formation and combustion process of sol–gel auto-combustion derived NiZn ferrite/SiO2 composites. Solid State Chem 177(11):4119–4125
Xiao T, Tang Y, Jia Z, Li D, Hu X, Li B, Luo L (2009) Self-assembled 3D flower-like Ni2+–Fe3+ layered double hydroxides and their calcined products. Nanotechnology 20(47):475603
Yazdani F, Seddigh M (2016) Magnetite nanoparticles synthesized by co-precipitation method: the effects of various iron anions on specifications. Mater Chem Phys 184:318–323
Zhang S, Zhao D (eds) (2017) Advances in magnetic materials: processing, properties, and performance. CRC Press, Boca Roton
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frolova, L.A. The mechanism of nickel ferrite formation by glow discharge effect. Appl Nanosci 9, 845–852 (2019). https://doi.org/10.1007/s13204-018-0767-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0767-z