Abstract
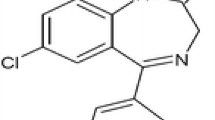
This work describes a novel method for the generation of a ketamine nano-delivery, to improve brain blood barrier permeability and increase drug therapeutic window as anaesthetic, analgesic and potential antidepressant. The approach herein described is based on ketamine-loaded poly-lactic-co-glycolic acid (PLGA) nanoparticles coupled to an apolipoprotein E (ApoE) peptide for delivery to the central nervous system. PLGA particles were synthesized with amount of drug, coupled with the ApoE peptide on the surface, and validated by physical characterization. The produced nanodevice showed a good colloidal stability in water, confirmed by zeta potential measurements, with a diameter in the range of 185–205 nm. The ketamine encapsulation was verified by liquid chromatography–mass spectrometry analyses obtaining an encapsulation efficiency up to 21.2 ± 3.54%. Once the occurrence of ApoE peptide functionalization was confirmed with fluorescence spectroscopy, the thermal stability and morphological information were obtained by differential scanning calorimetry and further dynamic light scattering measurements. The spherical shape and a rough nanoparticles surface were observed by atomic force microscopy. The reliability of this approach may be further developed as a protocol to be used to generate PLGA nanoparticles greater than 100 nm able to better penetrate blood brain barrier and release a neuroactive molecule at lower doses.







Similar content being viewed by others
References
Akinfiresoye L, Tizabi Y (2013) Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology 230(2):291–298. https://doi.org/10.1007/s00213-013-3153-2
Browne CA, Lucki I (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. https://doi.org/10.3389/fphar.2013.00161
Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK (2014) Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4(2):75–99. https://doi.org/10.1177/2045125313507739
Caffino L, Di Chio M, Giannotti G, Venniro M, Mutti A, Padovani L, Cheung D, Fumagalli GF, Yew DT, Fumagalli F, Chiamulera C (2016) The modulation of bdnf expression and signalling dissects the antidepressant from the reinforcing properties of ketamine: effects of single infusion vs. Chronic self-administration in rats. Pharmacol Res 104:22–30. https://doi.org/10.1016/j.phrs.2015.12.014
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62:35–41. https://doi.org/10.1016/j.neuropharm.2011.08.044
Eckert WA 3rd, Shoblock JR, McDuffie JE, Scott BP, Bonaventure P, Letavic MA, Vega J, Crowley T, Jiang X, Zannikos P, Singh JB, Chen G (2015) PK profiles of ketamine dosing regimens used in preclinical studies of its anti-depressant-like action. Program No. 425.04. 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online
Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology 231:3663–3676. https://doi.org/10.1007/s00213-014-3664-5
Han FY, Thurecht KJ, Lam AL, Whittaker AK, Smith MT (2015) Novel polymeric bioerodable microparticles for prolonged-release intrathecal delivery of analgesic agents for relief of intractable cancer-related pain. J Pharm Sci 104(7):2334–2344. https://doi.org/10.1002/jps.24497
Hans ML, Lowman AM (2002) Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci 6:319–327. https://doi.org/10.1016/s1359-0286(02)00117-1
Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76:970–976. https://doi.org/10.1016/j.biopsych.2014.03.026
Li L, Vlisides PE (2016) Ketamine: 50 years of modulating the mind. Front Hum Neurosci 10:612. https://doi.org/10.3389/fnhum.2016.00612
Makadia KH, Siegel SJ (2011) Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 3:1377–1397. https://doi.org/10.3390/polym3031377
Morgan CJ, Curran HV (2012) Independent scientific committee on drugs. Ketamine use: a review. Addiction 107(1):27–38. https://doi.org/10.1111/j.1360-0443.2011.03576.x
Portioli C, Bovi M, Benati D, Donini M, Perduca M, Romeo A, Dusi S, Monaco HL, Bentivoglio M (2017) Novel functionalization strategies of polymeric nanoparticles as carriers for brain medications. J Biomed Mater Res A 105:847–858. https://doi.org/10.1002/jbm.a.35961
Schifano F, Corkery J, Oyefeso A, Tonia T, Ghodse AH (2008) Trapped in the “K-hole”: overview of deaths associated with ketamine misuse in the UK (1993–2006). J ClinPsychopharmacol 28(1):114–116. https://doi.org/10.1097/jcp.0b013e3181612cdc
Shaffer CL, Osgood SM, Smith DL, Liu J, Trapa PE (2014) Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology 86:174–180. https://doi.org/10.1016/j.neuropharm.2014.07.008
Tamura T, Kita T, Nakagawa T, Endo T, Kim TS, Ishihara T, Mizushima Y, Higaki M, Ito J (2005) Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope 115(11):2000–2005. https://doi.org/10.1097/01.mlg.0000180174.81036.5a
Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L (2012) Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience 213:72–80. https://doi.org/10.1016/j.neuroscience.2012.03.052
Xu Y, Hackett M, Carter G, Loo C, Gálvez V, Glozier N, Glue P, Lapidus K, McGirr A, Somogyi AA, Mitchell PB, Rodgers A (2016) Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyv124
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) Nmdar inhibition-independent antidepressant actions of ketamine metabolites. Nat 533:481–486. https://doi.org/10.1038/nature17998
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. https://doi.org/10.1001/archpsyc.63.8.856
Zhao X, Venkata SL, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA Jr, Mager DE, Wainer IW (2012) Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J ClinPharmacol 74(2):304–314. https://doi.org/10.1111/j.1365-2125.2012.04198.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirano, S., Bovi, M., Romeo, A. et al. Ketamine nano-delivery based on poly-lactic-co-glycolic acid (PLGA) nanoparticles. Appl Nanosci 8, 655–663 (2018). https://doi.org/10.1007/s13204-018-0765-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0765-1