Abstract
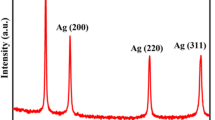
In this study, synthesis of Ag–Cu alloy bimetal nanoparticles anchored on high surface and porous ZnO using a facile, greener and low-cost aqeous bark extract of Aglaia roxburghiana for highly active, ultra-rapid and stable catalyst is performed. The nanocomposite was scrupulously characterized using UV–Vis spectrophotometer, X-ray diffraction, Raman spectrophotometer, high-resolution transmission electron microscope, selected area (electron) diffraction, scanning electron microscope with energy dispersive X-ray spectroscopy, and Fourier-transform infrared spectroscopy. The catalytic activity of the green synthesized Ag–Cu bimetal nanocomposite was evaluated in the reduction of 4-nitrophenol (4-NP), methylene blue (MB) and rhodamine B (Rh B) dyes. The different types of dye exhibited very high and effective catalytic activity within few seconds. The theoretical investigations reveal that the unique synergistic effect of Ag–Cu nanoparticles and immobilization over ZnO assists in the reduction of 4-NP, MB and Rh B. Loading and leaching of metal nanoparticles were obtained using inductively coupled plasma atomic emission spectroscopy. Moreover, the stable and efficient recyclability of nanocomposite by centrifugation after completion of the reaction was demonstrated. The results lead to the design different possible bimetal on ZnO with boosting and an effective catalyst for the environmental applications.











Similar content being viewed by others
References
Ali F, Khan SB, Kamal T, Alamry KA, Asiri AM, Sobahi TR (2017) Chitosan coated cotton cloth supported zero-valent nanoparticles: simple but economically viable, efficient and easily retrievable catalysts. Sci Rep 7:16957–16964
Astruc D, Lu F, Aranzaes JR (2005) Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Chem Int Ed 44:7852–7872
Bai X, Wang L, Zong R, Lv Y, Sun Y, Zhu Y (2013) Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir 29:3097–3105
Bhandary MJ, Chandrashekar KR (2011) Diversity and use of ethnomedicinal plants in coastal Karnataka, India. Indian J Tradit Knowl 10:528
Cao E, Duan W, Wang F, Wang A, Zheng Y (2017) Natural cellulose fiber derived hollow-tubular-oriented polydopamine: in-situ formation of Ag nanoparticles for reduction of 4-nitrophenol. Carbohydr Polym 158:44–50
Carnes CL, Stipp J, Klabunde KJ, Bonevich J (2002) Synthesis, characterization, and adsorption studies of nanocrystalline copper oxide and nickel oxide. Langmuir 18:1352–1359
Chang Z, Huo SJ, Zhang W, Fang J, Wang H (2017) The tunable and highly selective reduction products on Ag@Cu bimetallic catalysts towards CO2 electrochemical reduction reaction. J Phys Chem C 121:11368–11379
Chaudhary M, Doong RA, Kumar N, Tseng TY (2017) Ternary Au/ZnO/rGO nanocomposites electrodes for high performance electrochemical storage devices. Appl Surf Sci 420:118–128
Dhakshinamoorthy A, Asiri AM, Garcia H (2015) Metal–organic frameworks catalyzed C–C and C–heteroatom coupling reactions. Chem Soc Rev 44:1922–1947
Ernst JB, Muratsugu S, Wang F, Tada M, Glorius F (2016) Tunable heterogeneous catalysis: N-heterocyclic carbenes as ligands for supported heterogeneous Ru/K–Al2O3 catalysts to tune reactivity and selectivity. Am Chem Soc 138:10718–11021
Frindy S, El Kadib A, Lahcini M, Primo A, Garcia H (2016) Isotropic and oriented copper nanoparticles supported on graphene as aniline guanylation catalysts. ACS Catal 6:3863–3869
Gates BC (1995) Supported metal clusters: synthesis, structure, and catalysis. Chem Rev 95:511–522
Ghosh S, Goudar VS, Padmalekha KG, Bhat SV, Indi SS, Vasan HN (2012) ZnO/Ag nanohybrid: synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv 2:930–940
Gogoi A, Sarma KC (2017) Synthesis of the novel β-cyclodextrin supported CeO2 nanoparticles for the catalytic degradation of methylene blue in aqueous suspension. Mater Chem Phys 194:327–336
Gu S, Lu Y, Kaiser J, Albrecht M, Ballauff M (2015) Kinetic analysis of the reduction of 4-nitrophenol catalyzed by Au/Pd nanoalloys immobilized in spherical polyelectrolyte brushes. Phys Chem Chem Phys 17:28137–28143
Height MJ, Pratsinis SE, Mekasuwandumrong O, Praserthdam P (2006) Ag–ZnO catalysts for UV-photodegradation of methylene blue. Appl Catal A 63:305–312
Jayaprasad B, Sharavanan PS (2013) Overview of diabetics on Ayuverda. Int Res J Pharm 4(8):29–32. https://doi.org/10.7897/2230-8407.04804
Karunakaran C, Vinayagamoorthy P (2016) Magnetically recoverable Fe3O4-implanted Ag-loaded ZnO nanoflakes for bacteria-inactivation and photocatalytic degradation of organic pollutants. New J Chem 40:1845–1852
Khan FU, Khan SB, Kamal T, Asiri AM, Khan IU, Akhtarm K (2017) Novel combination of zero-valent Cu and Ag nanoparticles@ cellulose acetate nanocomposite for the reduction of 4-nitro phenol. Int J Biol Macromol 102:868–877
Khare CP (2008) Indian medicinal plants: an illustrated dictionary. Springer, Berlin. ISBN 978-0-387-70638-2
Khodadadi B, Bordbar M, Nasrollahzadeh M (2017) Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: catalytic activity for reduction of organic dyes. J Colloid Interface Sci 490:1–10
Kohantorabi M, Gholami MR (2017) M × Ni100 − x (M=Ag, and Co) nanoparticles supported on CeO2 nanorods derived from Ce–metal organic frameworks as an effective catalyst for reduction of organic pollutants: Langmuir–Hinshelwood kinetics and mechanism. New J Chem 41:10948–10958
Kumar B, Smita K, Cumbal L, Debut A, Angulo Y (2017) Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf. J Saudi Chem Soc 21(2017):S475–S480
Lei Y, Lee S, Low KB, Marshall CL, Elam JW (2016) Combining electronic and geometric effects of ZnO-promoted Pt nanocatalysts for aqueous phase reforming of 1-propanol. ACS Catal 6:3457–3460
Liang Y, Chen Z, Yao W, Wang P, Yu S, Wang X (2017) Decorating of Ag and CuO on Cu nanoparticles for enhanced high catalytic activity to the degradation of organic pollutants. Langmuir 33:7606–7614
Lunkenbein T, Schumann J, Behrens M, Schlögl R, Willinger MG (2015) Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal–support interactions. Angew Chem 127:4627–4631
Manjari G, Saran S, Arun T, Devipriya SP, Rao AVB (2017a) Facile Aglaia elaeagnoidea mediated synthesis of silver and gold nanoparticles: antioxidant and catalysis properties. J Clust Sci 28:1–16
Manjari G, Saran S, Arun T, Rao AV, Devipriya SP (2017b) Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. J Saudi Chem Soc 21:610–618
Manjari G, Saran S, Rao AV, Devipriya SP (2017c) Phytochemical screening of aglaia elaeagnoidea and their efficacy on antioxidant and antimicrobial growth. Int J Ayurveda Pharma Res 5:5–9
Mehrdad A, Massoumi B, Hashemzadeh R (2011) Kinetic study of degradation of Rhodamine B in the presence of hydrogen peroxide and some metal oxide. Chem Eng J 168:1073–1078
Momeni SS, Nasrollahzadeh M, Rustaiyan A (2016) Green synthesis of the Cu/ZnO nanoparticles mediated by Euphorbia prolifera leaf extract and investigation of their catalytic activity. J Colloid Interface Sci 472:173–179
Moulton MC, Braydich-Stolle LK, Nadagouda MN, Kunzelman S, Hussain SM, Varma RS (2010) Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale 2:763–770
Muellner AN, Greger H, Pannell CM (2009) Genetic diversity and geographic structure in Aglaia elaeagnoidea (Meliaceae, Sapindales), a morphologically complex tree species, near the two extremes of its distribution. Blumea Biodivers Evol Biogeogr Plants 54:207–216
Nasrollahzadeh M, Sajadi SM, Hatamifard A (2016) Waste chicken eggshell as a natural valuable resource and environmentally benign support for biosynthesis of catalytically active Cu/eggshell, Fe3O4/eggshell and Cu/Fe3O4/eggshell nanocomposites. Appl Catal B Environ 191:209–227
Patra S, Roy E, Madhuri R, Sharma PK (2016) Agar based bimetallic nanoparticles as high-performance renewable adsorbent for removal and degradation of cationic organic dyes. J Ind Eng Chem 33:226–238
Phan NT, Van Der Sluys M, Jones CW (2006) On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings–homogeneous or heterogeneous catalysis, a critical review. Adv Synth Catal 348:609–679
Rana S, Jonnalagadda SB (2017) A facile synthesis of Cu–Ni bimetallic nanoparticle supported organo functionalized graphene oxide as a catalyst for selective hydrogenation of p-nitrophenol and cinnamaldehyde. RSC Adv 7:2869–2879
Rout L, Kumar A, Dhaka RS, Reddy GN, Giri S, Dash P (2017) Bimetallic Au–Cu alloy nanoparticles on reduced graphene oxide support: synthesis, catalytic activity and investigation of synergistic effect by DFT analysis. Appl Catal A 538:107–122
Saleh R, Djaja NF (2014) Transition-metal-doped ZnO nanoparticles: synthesis, characterization and photocatalytic activity under UV light. Spectrochim Acta A 130:581–590
Sarma B, Deb SK, Sarma BK (2016) Photoluminescence and photocatalytic activities of Ag/ZnO metal-semiconductor heterostructure. Phys Conf Ser 765:012023
Schrader I, Warneke J, Backenköhler J, Kunz S (2015) Functionalization of platinum nanoparticles with l-proline: simultaneous enhancements of catalytic activity and selectivity. J Am Chem Soc 137:905–912
Sharma M, Hazra S, Basu S (2017) Synthesis of heterogeneous Ag–Cu bimetallic monolith with different mass ratios and their performances for catalysis and antibacterial activity. Adv Powder Technol 28:3085–3094
Somorjai GA, Contreras AM, Montano M, Rioux RM (2006) Clusters, surfaces, and catalysis. Proc Natl Acad Sci 103:10577–10583
Soundarrajan P, Sankarasubramanian K, Sampath M, Logu T, Sethuraman K, Ramamurthi K (2015) Cu ions induced reorientation of crystallite in ZnO nano/micro rod arrays thin films. Phys E Dimens Syst Nanostruct 71:56–63
Sun JK, Zhan WW, Akita T, Xu Q (2015) Toward homogenization of heterogeneous metal nanoparticle catalysts with enhanced catalytic performance: soluble porous organic cage as a stabilizer and homogenizer. J Am Chem Soc 137:7063–7066
Tuo Y, Liu G, Dong B, Yu H, Zhou J, Wang J, Jin R (2017) Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ Sci Pollut Res 24:5249–5258
Ucar A, Findik M, Gubbuk IH, Kocak N, Bingol H (2017) Catalytic degradation of organic dye using reduced graphene oxide–polyoxometalate nanocomposite. Mater Chem Phys 196:21–28
Valodkar M, Modi S, Pal A, Thakore S (2011) Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: a green approach. Mater Res Bull 46:384–389
Wang C, Ciganda R, Yate L, Moya S, Salmon L, Ruiz J, Astruc D (2017) RhAg/rGO nanocatalyst: ligand-controlled synthesis and superior catalytic performances for the reduction of 4-nitrophenol. J Mater Sci 52(2017):9465–9476
Wunder S, Polzer F, Lu Y, Mei Y, Ballauff M (2010) Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J Phys Chem C 114:8814–8820
Yang X, Zhong H, Zhu Y, Jiang H, Shen J, Huang J, Li C (2014) Highly efficient reusable catalyst based on silicon nanowire arrays decorated with copper nanoparticles. J Mater Chem A 2:9040–9047
Zhang B, Zhao B, Huang S, Zhang R, Xu P, Wang HL (2012) One-pot interfacial synthesis of Au nanoparticles and Au–polyaniline nanocomposites for catalytic applications. CrystEngComm 14:1542–1544
Zhang Gao H, Li J, Huang Y, Alsaedi A, Hayat T, Xu X, Wang X (2017) Rice husks as a sustainable silica source for hierarchical flower-like metal silicate architectures assembled into ultrathin nanosheets for adsorption and catalysis. J Hazard Mater 321:92–102
Acknowledgements
The authors are thankful to financial support from Pondicherry University. The authors acknowledge the Central Instrumentation Facility, Pondicherry University and Sophisticated Analytical Instrument Facility, STIC, Cochin for providing instrumentation facilities for characterization of the nanoparticles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gangarapu, M., Sarangapany, S., Suja, D.P. et al. Highly recyclable and ultra-rapid catalytic reduction of organic pollutants on Ag–Cu@ZnO bimetal nanocomposite synthesized via green technology. Appl Nanosci 8, 1123–1131 (2018). https://doi.org/10.1007/s13204-018-0753-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0753-5