Abstract
Silver nanoparticles (Ag-NPs) and its byproducts can spread pollution in aquatic habitat. Liver and gills are key target for toxicity. Oxidative stress, tissue alterations, and hemotoxicity are assumed to be associated with Ag-NPs in target animals. Cerium oxide nanoparticles (nano-ceria) show antioxidant potential in scavenging the free radicals generated in Ag-NP-induced oxidative stress. We determined ameliorated role of nano-ceria against Ag-NP-induced toxicity in fresh water Labeo rohita (L. rohita). Four groups were used in study including control, nano-ceria, Ag-NPs, and Ag-NPs + nano-ceria. Ag-NPs (30 mg l−1) and nano-ceria (50 µg kg−1) were given through water and prepared feed, respectively. The samples were taken after 28 days. Results demonstrated that pre-treatment of nano-ceria recovered L. rohita from Ag-NP-induced toxicity and oxidative stress. Nano-ceria pre-treatment actively mimics the activity of GST, GSH, CAT, and SOD. Furthermore, Ag-NPs’ treatment caused severe inflammation and necrosis in hepatic parenchyma which leaded to congestion of blood in hepatic tissues. Accumulation of a yellow pigment in hepatic tissue was also seen due to necrosis of affected cells. In nano-ceria pre-treatment, there was no congestion in hepatic tissue. Vacuolization of cells and necrosis in some area was recorded in nano-ceria pre-treated group, but the gill and hepatic tissue showed improvement against Ag-NP-induced damage. Nano-ceria pre-treatment also improved hematological parameters in Ag-NP-treated fish. This study concluded that Ag-NP-induced toxicity in treated fish and pre-treatment of nano-ceria show ameliorative role.
Similar content being viewed by others
Introduction
Ag-NPs are much rapidly growing class with 438 commercially available nanoproducts in international markets (Khan et al. 2015a; Wilson 2016). It is being used in batteries, photography, filters (Li et al. 2008), fabrics (Perelshtein et al. 2008), catalysts (Kumar et al. 2008), sensors (Schrand et al. 2008), textiles (Cohen et al. 2007; Ju-Nam and Lead 2008; Sondi and Salopek-Sondi 2004), medicine (Kirsner et al. 2001), in treatment of burns infections (Tredget et al. 1998), anticancer (Vergaro et al. 2011), antimicrobial (Du et al. 2018; Maillard and Hartemann 2013), and antifungal agent (Wright et al. 1999). Furthermore, the silver could also combine with other elements or compounds for various purposes. Excessive uses of these particles also increase discharge into aquatic habitats and exist in colloidal form. These particles persuade toxicity to invertebrate or vertebrate cell lines by production of reactive oxygen species (Ali 2014), apoptosis (Piao et al. 2011), reduced mitochondrial function (Ahamed et al. 2010), lipid peroxidation of membranes (Khan et al. 2017b; Zhornik et al. 2014), and oxidative stress marker depletion (Arora et al. 2009). Furthermore, Ag-NPs generate oxidative stress in target organisms (Devi et al. 2015; Khan et al. 2017b).
Labeo rohita belongs to widely cultured Indian major carps in south Asia including Bangladesh, India, Myanmar, Nepal, and Pakistan (Dahanukar 2010; Froese et al. 2016). This fish species inhibits freshwater rivers under a depth of 550 M and feed mainly on plankton (Wahab et al. 1995). As fish is the most important component of human food chain, therefore, it was recommended to use as animal model in toxicological studies. Gills are key organs for absorption and histo-pathological changes arise very quickly (Hawkins et al. 2015). When Ag-NPs enter in the animal’s body, they reach the liver through blood circulation and induce alterations (Khan et al. 2015a). Furthermore, this fish also shows oxidative stress (Khan et al. 2017b), alterations in blood hematology (Zutshi et al. 2010), genetic material (Khan et al. 2017a), and histology (Khan et al. 2017c) when exposed to toxic or environmental contaminates. These parameters can be used in studying the nature of new or novel compound (Khan et al. 2017a, b, c).
To cope with prooxidant level and maintain the healthy life, antioxidants are always necessary in diets (Asghar et al. 2018; Khan et al. 2016a; Zhang 2015). Nano-cerium is one of important antioxidant with long history of use as animal‘s feed additive (Khan et al. 2015a, b). Historically, Chinese community used cerium for increasing weight in cattle and egg production in breeders (Spivak et al. 2012). Currently, nano-ceria is extensively used in fuel as additive for better performance, polishing agent, radiation protecting, and antioxidant agent (Baker 2011; Karakoti et al. 2010; Park et al. 2007). Extensive application of nano-ceria raised concerned in researchers about long-term effects of exposures (Kumari et al. 2014). Still, insufficient information is available explaining the activities of nano-ceria in biological applications. Little attention was shifted towards unpredicted behavior of nano-ceria in aqueous and biological fluids (Shcherbakov et al. 2011). Review of published literature conformed the role of nano-ceria as antioxidant (Hosseini et al. 2015) and work as superoxide dismutase (SOD) (Korsvik et al. 2007), mimic catalase (CAT) (Pirmohamed et al. 2010), scavenging the hydroxyl radical (Xue et al. 2011), and nitric oxide free radicals (Dowding et al. 2012). In this study, the antioxidant potential of nano-ceria was tested against Ag-NP-induced toxicity and oxidative stress in 28 day treated L. rohita.
Materials and methods
Regents
All regents or chemicals of analytical grade were purchased from Merck and sigma Aldrich. Nano-ceria was in powder form with average size of 15.78 ± 5.56 nm. Ag-NPs’ particles were 16.59 ± 7.61 nm in size and prepared in the previous study by Khan et al. (2017b). The particles were characterized through SEM, TEM, XRD, EDS, and FT-IR analysis. The hydrodynamic size of Ag-NPs and nano-ceria (DLS measurements) was measured through Malvern Zetasizer using back-scattering detector. For DLS, stock solution was diluted with distilled water tenfold than original. Size was represented in intensity (%) of particles.
Experimental animal and laboratory conditions
The L. rohita (100 ± 5 g) as experimental model was obtained Punjab fishy department. The fishes were maintained in 40 l separate aquaria at room temperature (below 30 °C) and natural photoperiod. The dose for treatment for Ag-NPs was selected according to the previous study Khan et al. (2017b), where dose for nano-ceria was selected according to Estevez and Erlichman (2014). They used 30 mg kg−1 nano-ceria in diet of mice, but in our study, 50 µg kg−1 was used as higher concentration might produce toxicity in fish.
Two types of diets were formulated for study. Type 1 diet was consisted of only nutritional ingredients. Type 2 diet was consisted of 50 µg kg−1 nano-ceria along with nutritional ingredients. Fishes were divided into four treatments and three replicates, five fishes in each replicate. First treatment served as control and fed with type one diet. Type 2 diet was provided to second treatment. The treatment 3 was exposed 30 mg l−1 Ag-NPs along with feeding of type 1 diet. The treatment 4 was fed with type 2 diets and also exposed to 30 mg l−1Ag-NPs after 1 h of feeding. All treatments were fed twice a day times with specific diet for a period of 28 days. Water in each aquarium was replaced after 48 h.
Sampling
All sampling was done on the same day from randomly selected fishes of each replicate. Blood was taken via cardiac puncture with heparinized needle in EDTA tube. The gills and liver samples were preserved in 10% formalin solution.
Hematological analysis
Blood samples were analysed with M-20GP (MEDONIC Sweden) automatic hematology analyser. The activities of alanine amino transferase (ALT) and aspartate aminotransferase (AST) were measured with kinetic enzyme assay and expressed in IU/l.
The frequency of micronuclei was calculated through Giemsa stain smear formation as mentioned by Khan et al. (2017a) using formula:
Frequency of comet was measured with methodology of Singh et al. (1988). Damage was measured with tailed DNA migration (comet tail) in µm and frequency of comet. 100 cells per sample were analysed for damage.
Enzymatic analysis
The liver and gill tissue was washed separately with 1.15% KCl (ice cold), weighted 1 g, and homogenised with buffer (50 nM Tris–HCl, pH 7.4 and 1.15% KCl). The homogenised content was centrifuged (Sigma 2–16 k) for 20 min at 10,000 rpm. The supernatant was stored at − 20 °C after decantation.
The activity of catalase (CAT) was monitored by Bergmeyer (1965) using 5 mM hydrogen peroxide as substrate. Reaction solution was prepared by 50 mM peroxide with 50 mM potassium phosphate buffer. The H2O2 decomposition done by CAT was estimated through absorbance of UV spectrophotometer at 240 nm. Values were represented in in mol/mg sample protein.
Activity of superoxide dismutase (SOD) was estimated as U/mg protein with protocol of Payá et al. (1992) with some modification made by Peixoto and Pereira-Moura (2008). Activity assay was started in the presence of 10 mM nitrotetrazolium blue chloride (NBT) and 100 mM potassium phosphate buffer (pH 7.0). NBT acted as a detecting agent. About 0.023 U/mol of xanthine oxidase initiated the reaction when added to gill and liver extract at 25 °C. Then, enzyme activity was inhibited by 50% NBT.
The Glutathione S-transferase (GST) activity of was determined by Habig et al. (1974) with some modification. Reaction mixture was made with combination of 100 mM potassium phosphate buffer (2 ml), 10%, 1-chloro-2, 4 dinitrobenzene (100 ml), triton X-100, and 100 mM GST. The sample was added to start the reaction and absorbance was recorded at 340 nm. The activity of GST was expressed in mol/mg protein.
The Glutathione (GSH) activity was recorded using protocol of Jollow et al. (1974). In brief methodology, tissue extract was mixed with 500 µl 4% sulphosalicylic acid, incubated at 4 °C for 1 h, and centrifuged at 12,000 rpm for 15 min at 4 °C. About 0.4 ml of supernatant was initially mixed with 0.1 M potassium buffer (pH 7.4) and then with 0.4 ml DTNB. Colour of mixture was changed to yellow when GSH react with DTNB. Optical absorbance was recorded at 412 nm and expressed as µmol/g protein.
MDA (malondialdehyde) content was estimated for membrane lipid peroxidation (LPO). The methodology of Wilchek and Bayer (1990) was followed for this estimation. About 1 ml of sample extract was mixed with 2 ml of TBA–TCA–HCl, heated for 15 min in boiling water bath, and cooled at room temperature. The mixture was then centrifuged for 15 min at 4000 rpm for recording the absorbance at 535 nm against standard. The standard contained all the contents except sample. The molar concentration coefficient 1.5 × 10 M−1 cm−1 was used for MDA content and expressed in µmol/g protein.
Histological analysis was carried out through haematoxylin–eosin staining as mentioned by Khan et al. (2017c).
Statistical analysis
Data of three replicates were represented in the form of mean ± SD. ANOVA in general linear model was performed with IBM statistics (v. 20) to estimate the effects of treatment. Tukey’s test was used to compare the means. Propose of performing this test was to compare the control, nano-ceria, Ag-NP-treated, and Ag-NPs + nano-ceria-treated group. The values which were significantly different marked with different letters.
Results
Characterization of particles
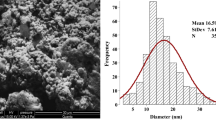
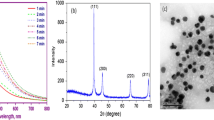
SEM image of Ag-NPs represents, and particles were spherical, grain like with agglomeration (Fig. 1). SEM image of CeO2-NPs also showed agglomeration of fine nano-powder. The histogram of Ag-NPs represents that most of particles were in 10–20 nm range with average size of 16.59 ± 7.61 nm. The histogram of CeO2-NPs shows maximum frequency between the ranges of 10–15 nm with an average size of 15.78 ± 5.56 nm (Fig. 2). TEM image represents spherical nature of sol and separated Ag-NPs. In electron diffraction analysis, ring patterns of electron diffraction shows (110), (200), (220), and (311) along growth direction which actually revealed crystalline, spherical, and face-centered cubic nature of synthesized Ag-NPs (Fig. 3). The hydrodynamic size was also measured through a Malvern Zeta sizer (nano-ZS) with back-scattering detector. Maximum particles were between 10 and 45 nm in case of Ag-NPs and 5–40 in nano-ceria samples. The DLS also presented that sampling dilutions with distilled water reduced agglomerations (Fig. 4). The FT-IR (Thermo Nicolet Avatar 380) spectra revealed coating and associated molecules with Ag-NPs. The peak spectrum at 3431.92 cm−1 was due to –N–H and O–H stretches. The second absorption band at 2842.03 cm−1 was due to C–H group vibrations. The third absorption band at 2382.15 cm−1 was due to absorbed or free water molecules vibrations. The fourth band at 1631.24 cm−1 represents C=C stretch. The absorption spectrum at 1392.12 cm−1 represents the attachment of amine group (C–N). The absorption bands at 1235.96 and 1054.82 cm−1 reveal C–N stretch vibrations. Over all the FT-IR spectra confirmed presence of amine interaction with Ag-NPs (Fig. 5). Distinct peaks in XRD spectra at 38.20θ, 44.40θ, 64.81θ, and 77.90θ confirm crystalline, face-centered, and cubic nature of Ag-NPs (Fig. 6). In Fig. 5, the characteristics peaks of XRD (111) at 29.5°, (200) at 33.3°, (220) at 47.6°, (311) at 57.5°, (222) at 59.1°, (400) at 64.4°, (331) at 76.6°, (420) at 79.3° and (422) at 88.5° of 2θ indicating the small, crystalline nature of particles and high purity of nano-ceria sample. High sample purity of both types of particles was also confirmed with EDX spectrum analysis. Spectra of both samples were only consisted of silver, cerium, and oxygen peaks. Impurity was not found in both recorded spectra (Fig. 7).
Ameliorative role in oxidative stress
Treatment of Ag-NPs negatively affected the activity of CAT in gill and liver tissue. A substantial increase in activity of CAT and SOD was indication of oxidative stress. The animal receiving pre-treatment of nano-ceria showed significant protection against CAT and SOD changes (at p < 0.05) due to active free radical scavenging and restored the activity of these enzymes (Fig. 8). Activity of GST increased with Ag-NPs’ treatment this might be due to conjugation of GSH with Ag+ ions. The treatment of nano-ceria reduced the conjugation of Ag+ and restored the activity of GST. Ag-NPs also increased the MDA contents in both tissues indicated the broken balance between antioxidant system and oxidative stress. Thus, radicals created increased lipid peroxidation of gill and liver tissue and hence increase the MDA content. Similar trend was in case of GSH. Activity of GSH was increased with increase of MDA content which indicated defensive mechanism of gill and liver against oxyradicals. Pre-treatment of nano-ceria reduced the MDA contents by scavenging oxyradicals and preventing the lipid peroxidation. Nano-ceria also restored activity of GSH. Non-significant difference of values between control and nano-ceria represents that nano-ceria alone posed no harmful effects and significant difference in values between Ag-NPs and Ag-NPs + nano-ceria showed antioxidant behavior of nano-ceria.
Ameliorative role in histopathology
Histological study revealed histo-protective effect of nano-ceria against Ag-NP-induced histopathology. Control and nano-ceria-treated liver section showed no visible alterations. Ag-NPs’ treatment caused severe inflammation and necrosis of hepatic parenchyma which leaded to congestion of blood in hepatic cells (black arrow). Furthermore, a yellow pigment was accumulated in hepatic tissue (bent arrow) which might be due to necrosis of affected effected cells (Fig. 9c). In case of nano-ceria pre-treatment, there was no congestion in hepatic tissue. Only vacuolization of cells (Black arrow) and accumulation of a yellow pigment (bent arrow) was recorded in hepatic tissue of nano-ceria pre-treated and Ag-NP-treated fish liver (Fig. 9d).
Ameliorative effective of nano-ceria in histopathology of liver in Ag-NPs treated L. rohita. Control (a) and nano-ceria (b) treated liver section showing no visible alteration. The 30 mg l−1 treatment of Ag-NPs produced alterations (c) marked with arrows, and nano-ceria pre-treatment showed improvement of gill tissue (d)
In gill section, inflammation of cartilage filament, separation of epithelial layer (white arrow), deformation of filament cartilage (black arrow and bent), and swelling and collapsing of gill cartilage (bent arrow) were seen in Ag-NP-treated fish (Fig. 10c, d). Nano-ceria-treated fish showed improvement in gill tissue. However, some alterations still occurred in the form of fused secondary lamellae (Black arrow), separated epithelial layer, cartilage filament inflammation (black bent arrow), and accumulation of macrophages (white arrow) in gill sections (Fig. 11a, b).
Ameliorative role in genotoxicity
Genoprotective nature of nano-ceria was confirmed through this study. There was non-significant difference of micronuclei between control and nano-ceria. Ag-NPs increased incidence of micronuclei in erythrocytes and nano-ceria pre-treatment reduced it in Ag-NP-treated fish. Furthermore, Ag-NP also increased the number of comet cells and DNA tail migration. However, nano-ceria significantly reduced the comet cells and DNA tail in Ag-NP-treated fishes (Table 1).
Ameliorated role in hematology
It was revealed that PCV, MCV, and MCH values were significantly reduced in Ag-NP-treated fish. However, the value of MCHC was significantly increased compared to control (Table 2). Furthermore, Ag-NPs’ treatment also reduced the level of HB, RBC, and increased WBCs and platelet count. Nano-ceria showed ameliorated role in Ag-NP-treated fish (Table 3). High value of ALT in Ag-NP-treated group represents onset of defensive mechanism of liver against Ag-NPs’ toxicity. ALT activity was significantly decreased in nano-ceria pre-treated fish. Similar trend was in AST activity (Table 4).
Discussion
Ag-NPs attracted much attention in toxicological studies. These particles can damage brain cell, liver, and stem cells. Some studies revealed that Ag-NPs can initiate oxidative stress (Asghar et al. 2016; Devi et al. 2015; Khan et al. 2017b) which is one of most important factor in toxicity (Khan et al. 2016b; Nel et al. 2006). This stress role in induction of apoptosis and damage to DNA (Simonian and Coyle 1996). This study recorded oxidative stress and changes in enzymatic level of gill and liver tissues in Ag-NP-treated L. rohita.
The nano-ceria is an evident antioxidant and ameliorative agent due to ability of redox potential (Heckert et al. 2008) and inactivation of ROS through scavenging (Spivak et al. 2012). It has Ce+3 and Ce+4 oxidation states with the ability to recycle. Oxygen valency is always created when oxidation state Ce+4 reduce to Ce+3 and forming Ce2O3 from Ce02 (Baalousha et al. 2010). This ability assisted ceria as an important antioxidant at physiological pH (Perez et al. 2008). Non-ceria directly act as antioxidant and block production of ROS by inhibiting program cell death or it activates the ROS defence system and reduce the level of ROS (Ciofani et al. 2014; Schubert et al. 2006). Multi-enzymatic mimetic properties of nano-ceria are supported by recent literature in biological systems (Buettner 2011; Jiao et al. 2012; Pirmohamed et al. 2010).
SOD deals with oxyradicals in antioxidant system and dismutase superoxide radicals into H2O2 and molecular oxygen. It is very sensitive in nature and responds quickly to environmental pollutants, therefore, extensively used as indicator of environmental pollutants. Increase level of SOD indicates onset of oxidative stress. However, reduced level indicates overwhelmed of antioxidant system due to excess ROS production (Rocca et al. 2015; Van der Oost et al. 2003). In this study, level of SOD was increased in Ag-NP-treated fishes. Pre-treatment of nano-ceria restored activity of SOD. Oxidation states Ce+3 and Ce+4 of nano-ceria are responsible to mimic SOD enzyme (Celardo et al. 2011). First ever evidence about this ability was found in study of Korsvik et al. (2007) how recorded SOD mimic activity in relation of Ce+3 and Ce+4 ratio (Korsvik et al. 2007; Kuchma et al. 2010).
CAT is another enzyme of prime importance in antioxidant system due to ability of converting H2O2 free radical into molecular oxygen and water. Significantly increased level of CAT was indication of oxidative stress. Pre-treatment of nano-ceria restored CAT activity. Pirmohamed et al. (2010) conducted amplex red assay and recorded CAT like activity of Ce+4 a in redox state. Furthermore, H2O2 was generated when nano-ceria acted as SOD. Luckily, nano-ceria acted as both CAT and SOD. H2O2 produces when body enters into dismutation process initiated by nano-ceria. This ability makes nano-ceria a strong antioxidant. Ce+3 and Ce+4 ratio, particles size, and pH are responsible for enzymatic properties of nano-ceria (Alili et al. 2011; Xue et al. 2011). Buffer species can also affects enzymatic properties of nano-ceria. The phosphate buffer can reduce SOD-mimetic activity, but increase CAT-mimetic activities (Alili et al. 2011).
GST is cytosolic in nature and has ability to catalyse conjugation of electrophilic substance with GSH. As GST detoxify exogenous substances, it is major target of toxic substances (Cheeke 1988). Ag-NPs’ treatment significantly changed the activity of GST compared to control and nano-ceria-treated group. This might be due to excess production of ROS (Khan et al. 2017b; Liu et al. 2014). Treatment of nano-ceria increased activity of GST compared to Ag-NP-treated group. Amin et al. (2011) showed similar increase in activity of GST with cerium oxide in monocrotaline-treated Sprague–dawley rats. Furthermore, level of MDA was increased in treated group. This increased level indicated that Ag-NPs induced oxyradicals’ production in liver and gills. Treatment of nano-ceria reduced lipid peroxidation of Ag-NP-treated group. It actually mimics the peroxidases activity and reduces the peroxidation of gill and liver similar to the Fenton reactions (Gao et al. 2007).
Retention of GSH is very essential for cellular homeostasis. Nano-ceria has GSH mimetic activity and could be explained on basis of scavenging of free radicals and direct exchange of electrons between ROS and electrons present on surface area of particles (Hochella et al. 2008). This capacity enables nano-ceria as highly reactive material in free radical scavenging and decreases the level of ROS creation and sustained GSH contents to homeostasis in biological systems (Niu et al. 2011).
In addition, nano-ceria also showed histo-protective role against Ag-NP-induced alterations in liver and gill tissues. Ag-NPs’ treatment caused severe inflammation and necrosis in hepatic parenchyma which leaded to congestion of blood in hepatic tissues. Furthermore, accumulation of yellow pigment in hepatic tissue was also seen due to necrosis of affected cells. In nano-ceria pre-treatment, there was no congestion in hepatic tissue. The vacuolization of cells and necrosis in some area was also occurred, but the hepatic tissue showed improvement against the Ag-NP-induced damage in the liver. Similar histo-protective effect was found in case of gill tissue. Tarnuzzer et al. (2005) demonstrated protective effect of nano-ceria against radiation-based cell death in human breast cell line. Furthermore, Niu et al. (2007) showed inhibitory effect of nano-ceria on development of cardiac dysfunction by removal of oxidative or ER stress and inhibition of inflammation.
Detection and level of aminotransferases in blood show damage to liver. These enzymes normally reside in hepatic cells and, however, spill into blood upon damage. Most common aminotransferases are ALT and AST which level increase in blood due to drug toxicity and cell death (Gontijo et al. 2003). Various chemical, biological, and physiological factors alter the activities of these enzymes producing disturbance in Kerb‘s cycle. Diminished level of Kreb’s cycle is also responsible for reduce level of intermediates which is then compensated by ALT and AST providing α ketoglutarate. This phenomenon increases blood serum level of both enzymes. In this study, a significantly increase in level of ALT and AST was recorded in Ag-NP-treated fish most probably due to damage in hepatic cells and inflammatory responses. This damage is initiated due to irritation of liver oxidant system which increases the level of free radicals (Wijnhoven et al. 2009). These radicals release ALT from hepatic cells into blood streams (Braydich-Stolle et al. 2005). The hepatic cells continuously detoxify toxic compounds. Any change in structure and metabolism changes animal physiological conditions. The present study confirms ameliorated role of nano-ceria in liver damage of Ag-NP-treated fish. El Shaer et al. (2017) found similar ameliorative role against isoproterenol-induced toxicity in male Wistar rats. Treatment of nano-ceria showed promising amelioration and decreased the level of ALT compared to Captopril drug. Like ALT, AST is also related to liver damage and its level increases in blood serum in case of toxicity to liver. However, AST is also found in skeletal muscle, kidney, heart muscle, red blood cells, and brain (Gaze 2007). In the present study, Ag-NPs increased the level of AST in treated fish. However, pre-treatment of nano-ceria significantly reduced the level. Bölükbaşı et al. (2016) used different doses to see the effect of dietary cerium oxide on egg lying performance of hen and found significant increase in egg quality parameters. They further found decreased level of AST in dose-dependent manner.
Ag-NPs produce significant genotoxicity in treated animals due to inflammation and oxidative stress (Asharani et al. 2009). Review of the literature advocated that these particles produce DNA damage in the form of double strands breaks, fusion, and fragmentation (AshaRani et al. 2008). This increased frequency of micronuclei, comet, and DNA tail migration. Nano-ceria pre-treatment significantly reduced frequency of micronuclei, comet, and DNA tail migration in Ag-NP-treated fish (Khan et al. 2017a). Rubio et al. (2016) found anti-genotoxic effect of CeO2-NPs in human epithelial lung cell line against oxidative stress inducing agent. Zhang et al. (2009) found inhibitory effect of cerium nitrate on tumour cell line. The 0.08 mm/l cerium nitrate shows less or no toxicity to normal cells, but inhibits the proliferation and growth of tumour cell line.
In Ag-NP-treated group, animal becomes anaemic and level of hemoglobin was decreased (Khan et al. 2017a). Furthermore, values of MCHC and MCH were different from control. Significantly, low level of these parameters was consequences of reduction in RBCs’ counts and haematocrit due to bleeding, deformation, damage to RBCs (Witeska and Kościuk 2003), haemolysis, or decrease in generation of RBCs (Di Giulio and Hinton 2008; Witeska and Kościuk 2003). Many researchers recorded decrease in level of MCH and MCHC due to exposure of metals in fresh water fishes (Vutukuru 2005). In general, fluctuations in studied blood parameters were due to defensive mechanism of body via erythropoiesis against toxicity (Vinodhini and Narayanan 2008). Decrease in level of MCHC and MCH was indication of toxicity of Ag-NPs in L. rohita. Variations in number of WBCs count was also recorded due to non-specific reaction of immune system against stress conditions (Stoskopf 1993; Vandebriel et al. 2014). Decrease in number of WBCs was suppression of immune response due to malfunctioning of hematopoietic system of treated fish (Adams 2002). In this study, WBCs count was increased due to non-specific immune system in response of low dose of particles. Pre-treatment of nano-ceria reduced alterations in hematological parameters due to reduction of oxidative stress. Adua et al. (2015) found dose-dependent increase in growth rate in black rabbits. Dietary 50–100 mg cerium oxide improved weight gain without any deleterious effect on blood parameters and blood serum.
Conclusions
Ag-NPs induce oxidative stress, hematological, and tissue alteration in L. rohita. The 28 day treatment produced fluctuations in antioxidant enzymes including GST, GSH, CAT, and SOD. These particles also produced severe inflammation of cartilage filament, separation of epithelial layer, deformation of filament cartilage, and swelling and collapsing of gill cartilage in treated fish. In liver section, Ag-NPs’ treatment caused severe inflammation and necrosis of hepatic parenchyma which leaded to congestion of blood in hepatic cells. Furthermore, a yellow pigment was accumulated in hepatic tissue which might be due to necrosis of affected effected cells. Ag-NPs treatment also produces fluctuations in blood parameters like reduction in PCV, MCV, MCH, Hb, and RBCs’ counts and increase in MCHC, WBC counts, and platelets’ counts. However, nano-ceria has ameliorative role due to multiple enzymes mimicking ability in response to higher oxidant level. This study confirmed nano-ceria as a novel antioxidant against oxidative stress, histological alterations and aided in recovery of antioxidant system.
References
Adams SM (2002) Biological indicators of aquatic ecosystem stress. American Fisheries Society, Bethesda
Adua O, Akinmuyisitana I, Gbore F (2015) Growth performance and blood profile of female rabbits fed dietary Cerium Oxide. J Biosci 21:69–75. https://doi.org/10.3329/jbs.v21i0.22521
Ahamed M, Alsalhi MS, Siddiqui MK (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411:1841–1848. https://doi.org/10.1016/j.cca.2010.08.016
Ali D (2014) Oxidative stress-mediated apoptosis and genotoxicity induced by silver nanoparticles in freshwater snail Lymnea luteola L. Biol Trace Elem Res 162:333–341. https://doi.org/10.1007/s12011-014-0158-6
Alili L, Sack M, Karakoti AS, Teuber S, Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S (2011) Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor–stroma interactions. Biomaterials 32:2918–2929. https://doi.org/10.1016/j.biomaterials.2010.12.056
Amin KA, Hassan MS, Awad ES, Hashem KS (2011) The protective effects of cerium oxide nanoparticles against hepatic oxidative damage induced by monocrotaline. Int J Nanomed 6:143–149. https://doi.org/10.2147/ijn.s15308
Arora S, Jain J, Rajwade J, Paknikar K (2009) Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol 236:310–318. https://doi.org/10.1016/j.taap.2009.02.020
Asghar MS, Quershi NA, Jabeen F, Shakeel M, Khan MS (2016) Genotoxicity and oxidative stress analysis in the Catla catla treated with ZnO NPs J Bio. Environ Sci 8:91–104
Asghar MS, Qureshi NA, Jabeen F, Khan MS, Shakeel M, Chaudhry AS (2018) Ameliorative effects of selenium in ZnO NP-induced oxidative stress and hematological alterations in Catla catla. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1299-9
AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S (2008) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS nano 3:279–290. https://doi.org/10.1021/nn800596w
Asharani P, Hande MP, Valiyaveettil S (2009) Anti-proliferative activity of silver nanoparticles. BMC Cell Biol 10:65. https://doi.org/10.1186/1471-2121-10-65
Baalousha M, Le Coustumer P, Jones I, Lead J (2010) Characterisation of structural and surface speciation of representative commercially available cerium oxide nanoparticles. Environ Chem 7:377–385. https://doi.org/10.1071/en10003
Baker CH (2011) Radiation protection with nanoparticles. Nanomed Health Dis. https://doi.org/10.1201/b11076-15
Bergmeyer HU (1965) Principles of enzymatic analysis. In: Methods of enzymatic analysis. Elsevier, p 3–13. https://doi.org/10.1016/b978-0-12-395630-9.50008-6
Bölükbaşı S, Al-sagan A, Ürüşan H, Erhan M, Durmuş O, Kurt N (2016) Effects of cerium oxide supplementation to laying hen diets on performance, egg quality, some antioxidant enzymes in serum and lipid oxidation in egg yolk. J Anim Physiol Anim Nutr 100:686–693
Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann M-C (2005) In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci 88:412–419. https://doi.org/10.1093/toxsci/kfi256
Buettner GR (2011) Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem 11:341. https://doi.org/10.2174/187152011795677544
Celardo I, De Nicola M, Mandoli C, Pedersen JZ, Traversa E, Ghibelli L (2011) Ce3 + ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 5:4537–4549. https://doi.org/10.1021/nn200126a
Cheeke P (1988) Toxicity and metabolism of pyrrolizidine alkaloids. J Anim Sci 66:2343–2350. https://doi.org/10.2527/jas1988.6692343x
Ciofani G, Genchi GG, Mazzolai B, Mattoli V (2014) Transcriptional profile of genes involved in oxidative stress and antioxidant defense in PC12 cells following treatment with cerium oxide nanoparticles. Biochim Biophys Acta 1840:495–506. https://doi.org/10.1016/j.bbagen.2013.10.009
Cohen MS, Stern JM, Vanni AJ, Kelley RS, Baumgart E, Field D, Libertino JA, Summerhayes IC (2007) In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg Infect 8:397–404. https://doi.org/10.1089/sur.2006.032
Dahanukar N (2010) Labeo rohita. The IUCN red list of threatened species
Devi GP, Ahmed KBA, Varsha MS, Shrijha B, Lal KS, Anbazhagan V, Thiagarajan R (2015) Sulfidation of silver nanoparticle reduces its toxicity in zebrafish. Aquat Toxicol 158:149–156. https://doi.org/10.1016/j.aquatox.2014.11.007
Di Giulio RT, Hinton DE (2008) The toxicology of fishes. Crc Press, Boca Raton. https://doi.org/10.1201/9780203647295
Dowding JM, Dosani T, Kumar A, Seal S, Self WT (2012) Cerium oxide nanoparticles scavenge nitric oxide radical (˙ NO). Chem Commun 48:4896–4898. https://doi.org/10.1039/c2cc30485f
Du Y, Huang Z, Wu S, Xiong K, Zhang X, Zheng B, Nadimicherla R, Fu R, Wu D (2018) Preparation of versatile yolk-shell nanoparticles with a precious metal yolk and a microporous polymer shell for high-performance catalysts and antibacterial agents. Polymer 137:195–200. https://doi.org/10.1016/j.polymer.2017.12.069
El Shaer SS, Salaheldin TA, Saied NM, Abdelazim SM (2017) In vivo ameliorative effect of cerium oxide nanoparticles in isoproterenol-induced cardiac toxicity. Exp Toxicol Pathol. https://doi.org/10.1016/j.etp.2017.03.001
Estevez AY, Erlichman JS (2014) The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy. Nanomedicine 9:1437–1440. https://doi.org/10.2217/nnm.14.87
Froese R, Winker H, Gascuel D, Sumalia UR, Pauly D (2016) Minimizing the impact of fishing. Fish Fish 17:785–802. https://doi.org/10.1111/faf.12146
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. https://doi.org/10.1038/nnano.2007.260
Gaze DC (2007) The role of existing and novel cardiac biomarkers for cardioprotection. Curr Opin Investig Drugs 8:711–717
Gontijo ÁMdMC, Barreto RE, Speit G, Reyes VAV, Volpato GL, Salvadori DMF (2003) Anesthesia of fish with benzocaine does not interfere with comet assay results. Mutat Res Gen Tox En 534:165–172. https://doi.org/10.1016/s1383-5718(02)00276-0
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hawkins AD, Thornton C, Kennedy AJ, Bu K, Cizdziel J, Jones BW, Steevens JA, Willett KL (2015) Gill histopathologies following exposure to nanosilver or silver nitrate. J Toxicol Environ Health Part A 78:301–315. https://doi.org/10.1080/15287394.2014.971386
Heckert EG, Karakoti AS, Seal S, Self WT (2008) The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29:2705–2709. https://doi.org/10.1016/j.biomaterials.2008.03.014
Hochella MF, Lower SK, Maurice PA, Penn RL, Sahai N, Sparks DL, Twining BS (2008) Nanominerals, mineral nanoparticles, and earth systems. Science 319:1631–1635. https://doi.org/10.1126/science.1141134
Hosseini A, Sharifi AM, Abdollahi M, Najafi R, Baeeri M, Rayegan S, Cheshmehnour J, Hassani S, Bayrami Z, Safa M (2015) Cerium and yttrium oxide nanoparticles against lead-induced oxidative stress and apoptosis in rat hippocampus. Biol Trace Elem Res 164:80–89. https://doi.org/10.1007/s12011-014-0197-z
Jiao X, Song H, Zhao H, Bai W, Zhang L, Lv Y (2012) Well-redispersed ceria nanoparticles: promising peroxidase mimetics for H2O2 and glucose detection Anal. Methods 4:3261–3267. https://doi.org/10.1039/c2ay25511a
Jollow D, Mitchell J, Na Zampaglione, Gillette J (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169. https://doi.org/10.1159/000136485
Ju-Nam Y, Lead JR (2008) Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ 400:396–414. https://doi.org/10.1016/j.scitotenv.2008.06.042
Karakoti A, Singh S, Dowding JM, Seal S, Self WT (2010) Redox-active radical scavenging nanomaterials. Chem Soc Rev 39:4422–4432. https://doi.org/10.1039/b919677n
Khan MS, Jabeen F, Asghar MS, Qureshi NA, Shakeel M, Noureen A, Shabbir S (2015a) Role of nao-ceria in the amelioration of oxidative stress: current and future applications in medicine Int. J Biosci 6:89–109. https://doi.org/10.12692/ijb/6.8.89-109
Khan MS, Jabeen F, Qureshi NA, Asghar MS, Shakeel M, Noureen A (2015b) Toxicity of silver nanoparticles in fish: a critical review. J Bio Environ Sci 6:211–227
Khan MS, Quershi NA, Jabeen F, Asghar MS, Shakeel M (2016a) Analysis of minerals profile, phenolic compounds and potential of Garlic (Allium sativum) as antioxidant scavenging the free radicals. Int J Biosci 8:72–82. https://doi.org/10.12692/ijb/8.4.72-82
Khan MU, Qurashi NA, Khan MS, Jabeen F, Umar A, Yaqoob J, Wajid M (2016b) Generation of reactive oxygen species and their impact on the health related parameters: a critical review Int. J Biosci 9:303–323. https://doi.org/10.12692/ijb/9.1.303-323
Khan MS, Qureshi NA, Jabeen F (2017a) Assessment of toxicity in fresh water fish Labeo rohita treated with silver nanoparticles. Appl Nanosci. https://doi.org/10.1007/s13204-017-0559-x
Khan MS, Qureshi NA, Jabeen F, Asghar MS, Shakeel M, Fakhar-e-Alam M (2017b) Eco-friendly synthesis of silver nanoparticles through economical methods and assessment of toxicity through oxidative stress analysis in the Labeo rohita. Biol Trace Elem Res 176:416–428. https://doi.org/10.1007/s12011-016-0838-5
Khan MS, Qureshi NA, Jabeen F, Shakeel M, Asghar MS (2017c) Assessment of waterborne amine-coated silver nanoparticle (Ag-NP)-induced toxicity in Labeo rohita by histological and hematological profiles. Biol Trace Elem Res 182:1–10. https://doi.org/10.1007/s12011-017-1080-5
Kirsner R, Orsted H, Wright B (2001) Matrix metalloproteinases in normal and impaired wound healing: a potential role of nanocrystalline silver. Wounds 13:4–12
Korsvik C, Patil S, Seal S, Self WT (2007) Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun 10:1056–1058. https://doi.org/10.1039/b615134e
Kuchma MH, Komanski CB, Colon J, Teblum A, Masunov AE, Alvarado B, Babu S, Seal S, Summy J, Baker CH (2010) Phosphate ester hydrolysis of biologically relevant molecules by cerium oxide nanoparticles. Nanomedicine 6:738–744. https://doi.org/10.1016/j.nano.2010.05.004
Kumar PSS, Sivakumar R, Anandan S, Madhavan J, Maruthamuthu P, Ashokkumar M (2008) Photocatalytic degradation of acid Red 88 using Au–TiO 2 nanoparticles in aqueous solutions. Water Res 42:4878–4884. https://doi.org/10.1016/j.watres.2008.09.027
Kumari M, Kumari SI, Grover P (2014) Genotoxicity analysis of cerium oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral administration. Mutagen 29:467–479. https://doi.org/10.1093/mutage/geu038
Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, Alvarez PJ (2008) Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res 42:4591–4602. https://doi.org/10.1016/j.watres.2008.08.015
Liu C, Yang X, Yao Y, Huang W, Sun W, Ma Y (2014) Determination of antioxidant activity in garlic (Allium sativum) extracts subjected to boiling process in vitro. J Food Nutr Res 2:383–387. https://doi.org/10.12691/jfnr-2-7-9
Maillard J-Y, Hartemann P (2013) Silver as an antimicrobial: facts and gaps in knowledge. Crit Rev Microbiol 39:373–383. https://doi.org/10.3109/1040841x.2012.713323
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627. https://doi.org/10.1126/science.1114397
Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE (2007) Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res 73:549–559. https://doi.org/10.1016/j.cardiores.2006.11.031
Niu J, Wang K, Kolattukudy PE (2011) Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharm Exp Ther 338:53–61. https://doi.org/10.1124/jpet.111.179978
Park B, Martin P, Harris C, Guest R, Whittingham A, Jenkinson P, Handley J (2007) Initial in vitro screening approach to investigate the potential health and environmental hazards of Envirox™-a nanoparticulate cerium oxide diesel fuel additive. Particle Fibre Toxicol 4:4–12. https://doi.org/10.1186/1743-8977-4-12
Payá M, Halliwell B, Hoult J (1992) Interactions of a series of coumarins with reactive oxygen species: scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem Pharmacol 44:205–214. https://doi.org/10.1016/0006-2952(92)90002-z
Peixoto AL, Pereira-Moura MVL (2008) A new genus of Monimiaceae from the Atlantic Coastal Forest in south-eastern Brazil. Kew Bull 63:137–141. https://doi.org/10.1007/s12225-007-9004-8
Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A (2008) Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology 19:245705. https://doi.org/10.1088/0957-4484/19/24/245705
Perez JM, Asati A, Nath S, Kaittanis C (2008) Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 4:552–556. https://doi.org/10.1002/smll.200700824
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett 201:92–100. https://doi.org/10.1016/j.toxlet.2010.12.010
Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JE, Seal S, Self WT (2010) Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun 46:2736–2738. https://doi.org/10.1039/b922024k
Rocca A, Moscato S, Ronca F, Nitti S, Mattoli V, Giorgi M, Ciofani G (2015) Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomedicine 11:1725–1734. https://doi.org/10.1016/j.nano.2015.05.001
Rubio L, Annangi B, Vila L, Hernández A, Marcos R (2016) Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch Toxicol 90:269–278. https://doi.org/10.1007/s00204-015-1468-y
Schrand AM, Braydich-Stolle LK, Schlager JJ, Dai L, Hussain SM (2008) Can silver nanoparticles be useful as potential biological labels? Nanotechnology 19:235104. https://doi.org/10.1088/0957-4484/19/23/235104
Schubert D, Dargusch R, Raitano J, Chan S-W (2006) Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun 342:86–91. https://doi.org/10.1016/j.bbrc.2006.01.129
Shcherbakov A, Ivanov V, Zholobak N, Ivanova O, Krysanov EY, Baranchikov A, Spivak NY, Tretyakov YD (2011) Nanocrystalline ceria based materials—Perspectives for biomedical application. Biophysics 56:987–1004. https://doi.org/10.1134/s0006350911060170
Simonian N, Coyle J (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106. https://doi.org/10.1146/annurev.pa.36.040196.000503
Singh N, Mccoy M, Tice R, Schneider E (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182. https://doi.org/10.1016/j.jcis.2004.02.012
Spivak NY, Zholobak N, Shcherbakov A, Antonovitch G, Yanchiy R, Ivanov V, Tretyakov Y (2012) Ceria nanoparticles boost activity of aged murine oocytes. Nano Biomed Eng 4:188–194. https://doi.org/10.5101/nbe.v4i4.p188-194
Stoskopf KM (1993) Fish medicine, 1st edn. Saunders Co., Philadelphia. https://doi.org/10.2307/1447015
Tarnuzzer RW, Colon J, Patil S, Seal S (2005) Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett 5:2573–2577. https://doi.org/10.1021/nl052024f
Tredget EE, Shankowsky HA, Groeneveld A, Burrell R (1998) A matched-pair, randomized study evaluating the efficacy and safety of Acticoat* silver-coated dressing for the treatment of burn wounds. J Burn Care Res 19:531–537. https://doi.org/10.1097/00004630-199811000-00013
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharm 13:57–149. https://doi.org/10.1016/s1382-6689(02)00126-6
Vandebriel RJ, Tonk EC, de la Fonteyne-Blankestijn LJ, Gremmer ER, Verharen HW, van der Ven LT, van Loveren H, de Jong WH (2014) Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part Fibre Toxicol 11:1–9. https://doi.org/10.1186/1743-8977-11-21
Vergaro V, Scarlino F, Bellomo C, Rinaldi R, Vergara D, Maffia M, Baldassarre F, Giannelli G, Zhang X, Lvov YM, Leporatti S (2011) Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv Drug Deliv Rev 63:847–864. https://doi.org/10.1016/j.addr.2011.05.007
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int J Environ Sci Technol 5:179–182. https://doi.org/10.1007/bf03326011
Vutukuru S (2005) Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeo rohita. Int J Environ Res Publ Health 2:456–462. https://doi.org/10.3390/ijerph2005030010
Wahab M, Ahmed Z, Islam MA, Haq M, Rahmatullah S (1995) Effects of introduction of common carp, Cyprinus carpio (L.), on the pond ecology and growth of fish in polyculture. Aquac Res 26:619–628. https://doi.org/10.1111/j.1365-2109.1995.tb00953.x
Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG, Heugens EH, Roszek B, Bisschops J, Gosens I, Van De Meent D (2009) Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3:109–138. https://doi.org/10.1080/17435390902725914
Wilchek M, Bayer EA (1990) Introduction to avidin-biotin technology. Methods Enzymol 184:5–13. https://doi.org/10.1016/0076-6879(90)84256-g
Wilson Wd (2016) Nanotechnology consumer product inventory. http://www.nanotechproject.org/cpi/about/analysis. Accessed 28 April 2016
Witeska M, Kościuk B (2003) The changes in common carp blood after short-term zinc exposure. Environ Sci Pollut R 10:284–286. https://doi.org/10.1065/espr2003.07.161
Wright J, Lam K, Hansen D, Burrell R (1999) Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control 27:344–350. https://doi.org/10.1016/s0196-6553(99)70055-6
Xue Y, Luan Q, Yang D, Yao X, Zhou K (2011) Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C 115:4433–4438. https://doi.org/10.1021/jp109819u
Zhang X (2015) Tea and cancer prevention. J Cancer Res Updates 4:65–73. https://doi.org/10.6000/1929-2279.2015.04.02.4
Zhang GY, Zhao HJ, Xin ZJ, Qu JL, Wang BG, Wang H (2009) Effect of cytotoxicity on normal cells and anti-tumor activity on tumor cells of cerium nitrate in vitro. J Xian Jiaotong Univ 6:017
Zhornik E, Baranova L, Drozd E, Sudas M, Chau N, Buu N, Dung T, Chizhik S, Volotovski I (2014) Silver nanoparticles induce lipid peroxidation and morphological changes in human lymphocytes surface. Biophysics 59:380–386. https://doi.org/10.1134/s0006350914030282
Zutshi B, Prasad SGR, Nagaraja R (2010) Alteration in hematology of Labeo rohita under stress of pollution from Lakes of Bangalore, Karnataka, India. Environ Monit Assess 168:11–19. https://doi.org/10.1007/s10661-009-1087-2
Acknowledgements
Authors are grateful to department of Zoology faculty and staff members for providing support. This research was done in university own resources and does not receive any grant or funds in any form. This research was the part of author Ph.D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no conflict of interest in any form.
Ethical approval
All adopted procedures and use of fishes in this study were in agreement with ethical standards in research of university, Government College University Faisalabad, Pakistan.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, M.S., Qureshi, N.A. & Jabeen, F. Ameliorative role of nano-ceria against amine coated Ag-NP induced toxicity in Labeo rohita. Appl Nanosci 8, 323–337 (2018). https://doi.org/10.1007/s13204-018-0733-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0733-9