Abstract
Salt stress is a major environmental constraint that limits growth and nitrogen-fixation in legumes. Role of putrescine (Put) and arbuscular mycorrhiza (AM) in improving functional efficiency of legumes has gained importance in recent years. Present investigations assessed the impact of Put (1 mM) seed priming and/or Rhizophagus irregularis inoculation on growth, mycorrhizal and rhizobial symbioses with an emphasis to correlate the same with nodular Put metabolism in pigeonpea (Cajanus cajan L.) genotypes (Tolerant-Pusa 2001 and Sensitive-Pusa 991) under salt stress. Salinity declined plant biomass, with greater negative effects on roots than shoots which reduced the mycorrhizal colonization as well as nodulation ability of Sinorhizobium fredii AR-4. The decline in nitrogen-fixing efficiency could be correlated with increase in Na+ concentrations in roots as well as nodules. Salinity reduced endogenous Put by increasing diamine oxidase (DAO) as well as decreasing arginine decarboxylase (ADC) as well as ornithine decarboxylase (ODC) activities in nodules. Put priming enhanced per cent mycorrhizal colonization which further increased the rhizobial symbiotic efficiency, more in Pusa 2001 than Pusa 991. Both Put priming and AM inoculations reduced Na+ uptake and improved nutrient status especially P in both underground organs, with AM more effective than Put. The further decline in Na+ uptake was recorded when both amendments were given together which enhanced nitrogen-fixing ability of nodules by modulating anabolic and catabolic enzyme activities responsible for Put biosynthesis. Hence, +Put+AM can be used as an effective strategy to improve symbiotic potential and arrest nodule senescence in pigeonpea under salt stress.





Similar content being viewed by others
References
Abd-Alla MH, El-Enany AWE, Nafady NA, Khalaf DM, Morsy FM (2014) Synergistic interaction of Rhizobium leguminosarum bv. Viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol Res 169(1):49–58
Ali RM (2000) Role of putrescine in salt tolerance of Atropa belladonna plant. Plant Sci 152(2):173–179
Ali Z, Salam A, Azhar FM, Khan IA (2007) Genotypic variation in salinity tolerance among spring and winter wheat (Triticum aestivum L.) accessions. South Afr J Bot 73:70–75
Allen SF, Grimshaw HF, Rowl AB (1984) Chemical analysis. In: Moor PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 185–344
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44(5):384–395
Becerra-Rivera VA, Bergström E, Thomas-Oates J, Dunn MF (2018) Polyamines are required for normal growth in Sinorhizobium meliloti. Microbiology 164(4):600–613
Benavides MP, Aizencang G, Tomaro ML (1997) Polyamines in Helianthus annuus L. during germination under salt stress. J Plant Growth Regul 16(4):205–211
Braeken K, Daniels R, Vos K, Fauvart M, Bachaspatimayum D, Vanderleyden J, Michiels J (2008) Genetic determinants of swarming in Rhizobium etli. Microb Ecol 55(1):54–64
Bruning B, Rozema J (2013) Symbiotic nitrogen fixation in legumes: perspectives for saline agriculture. Environ Exp Bot 92:134–143
Céccoli G, Ramos JC, Ortega LI, Acosta JM, Perreta MG (2011) Salinity induced anatomical and morphological changes in Chloris gayana Kunth roots. Biocell 35(1):9–17
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58(15–16):4245–4255
Cheng Y, Ma W, Li X, Miao W, Zheng L, Cheng B (2012) Polyamines stimulate hyphal branching and infection in the early stage of Glomus etunicatum colonization. World J Microbiol Biotechnol 28(4):1615–1621
Cicatelli A, Lingua G, Todeschini V, Biondi S, Torrigiani P, Castiglione S (2010) Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot 106(5):791–802
Djanaguiraman M, Prasad PV (2013) Effects of salinity on ion transport, water relations and oxidative damage. In: Ecophysiology and responses of plants under salt stress. Springer, New York, pp 89–114
Do PT, Drechsel O, Heyer AG, Hincha DK, Zuther E (2014) Changes in free polyamine levels, expression of polyamine biosynthesis genes, and performance of rice cultivars under salt stress: a comparison with responses to drought. Front Plant Sci 5:182
Egamberdieva D, Li L, Lindström K, Räsänen LA (2016) A synergistic interaction between salt-tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis fish.) under salt stress. Appl Microbiol Biotechnol 100(6):2829–2841
El Ghachtouli N, Martin-Tanguy J, Paynot M, Gianinazzi S (1996) First-report of the inhibition of arbuscular mycorrhizal infection of Pisum sativum by specific and irreversible inhibition of polyamine biosynthesis or by gibberellic acid treatment. FEBS Lett 385(3):189–192
El Ghachtouli N, Paynot M, Morandi D, Martin-Tanguy J, Gianinazzi S (1995) The effect of polyamines on endomycorrhizal infection of wild-type Pisum sativum, cv. Frisson (nod+ myc+) and two mutants (nod− myc+ and nod− myc−). Mycorrhiza 5(3):189–192
Estrada B, Aroca R, Maathuis FJ, Barea JM, Ruiz-Lozano JM (2013) Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ 36(10):1771–1782
Evelin H, Giri B, Kapoor R (2012) Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22(3):203–217
Fahmi AI, Nagaty HH, Eissa RA, Hassan MM (2011) Effects of salt stress on some nitrogen fixation parameters in faba bean. Pak J Biol Sci 14(6):385
FAOSTAT (2018) http://www.fao.org/faostat/en/#data/QC [Accessed 23 Oct. 2018]
Fariduddin Q, Mir BA, Yusuf M, Ahmad A (2014) 24-Epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 52(3):464–474
Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, Migdadi HM, Alghamdi SS, Siddique KH (2017) Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem 118:199–217
Flores HE, Galston A (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69(3):701–706
Fujihara S, Abe H, Minakawa Y, Akao S, Yoneyama T (1994) Polyamines in nodules from various plant-microbe symbiotic associations. Plant Cell Physiol 35(8):1127–1134
Garg N, Bhandari P (2016) Silicon nutrition and mycorrhizal inoculations improve growth, nutrient status, K+/Na+ ratio and yield of Cicer arietinum L. genotypes under salinity stress. Plant Growth Regul 78(3):371–387
Garg N, Bharti A (2018) Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza 28(8):727–746
Garg N, Manchanda G (2008) Effect of arbuscular mycorrhizal inoculation on salt-induced nodule senescence in Cajanus cajan (pigeonpea). J Plant Growth Regul 27(2):115–124. https://doi.org/10.1007/s00344-007-9038-z
Garg N, Singla P (2016) Stimulation of nitrogen fixation and trehalose biosynthesis by naringenin (Nar) and arbuscular mycorrhiza (AM) in chickpea under salinity stress. Plant Growth Regul 80(1):5–22
Giovannetti M, Mosse B (1980) Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331(1–2):313–327
Hammer EC, Nasr H, Pallon J, Olsson PA, Wallander H (2011) Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 21(2):117–129
Hammer EC, Rillig MC (2011) The influence of different stresses on glomalin levels in an arbuscular mycorrhizal fungus—salinity increases glomalin content. PLoS One 6(12):e28426
Hartree EF (1957) Haematin compounds. In: Paech K, Tracey MV (eds) Modern methods of plant analysis. Springer, Berlin, pp 197–245
Hasanuzzaman M, Nahar K, Fujita M, Ahmad P, Chandna R, Prasad MNV, Ozturk M (2013) Enhancing plant productivity under salt stress: relevance of poly-omics. In: Salt Stress in Plants. Springer, New York, pp 113–156
Herdina JA, Silsbury JH (1990) Estimating nitrogenase activity of faba bean (Vicia faba) by acetylene reduction (AR) assay. Aust J Plant Physiol 17(5):489–502
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hršelová H, Gryndler M (2000) Effect of spermine on proliferation of hyphae of Glomus fistulosum, an arbuscular mycorrhizal fungus, in maize roots. Folia Microbiol (Praha) 45(2):167
IAB (2000) Indian Agriculture in Brief, 27th edn. Agriculture Statistics Division, Ministry of Agriculture, Govt. of India, New Delhi
Iqbal M, Ashraf M (2006) Wheat seed priming in relation to salt tolerance: growth, yield and levels of free salicylic acid and polyamines. Ann Bot Fenn 43(4):250–259
Ismail AM, Horie T (2017) Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu Rev Plant Biol 68(1):405–434. https://doi.org/10.1146/annurev-arplant-042916-040936
Jackson ML (1973) Soil chemical analysis. Published by Printice Hall, New Delhi, p 485
Jiménez Bremont JF, Marina M, Guerrero-González MD, Rossi FR, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz OA, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95
Kenenil A, Assefa F, Prabu PC (2010) Characterization of acid and salt tolerant rhizobial strains isolated from faba bean fields of Wollo northern Ethiopia. J Agric Sci Technol 12:365–376
Kormanik PP, McGraw AC (1982) Quantification of vesicular-arbuscular mycorrhizae in plant roots. In: Schenk NC (ed) Methods and principles of mycorrhizal research. APS Press, Minneapolis
Krishnamurthy R, Bhagwat KA (1989) Polyamines as modulators of salt tolerance in rice cultivars. Plant Physiol 91(2):500–504
Kumar V, Khare T (2015) Individual and additive effects of Na+ and cl− ions on rice under salinity stress. Arch Agron Soil Sci 61(3):381–395
Lahiri K, Chattopadhyay S, Ghosh B (2004) Correlation of endogenous free polyamine levels with root nodule senescence in different genotypes in Vigna mungo L. J Plant Physiol 161(5):563
Legocka J, Sobieszczuk-Nowicka E (2012) Sorbitol and NaCl stresses affect free, microsome-associated and thylakoid-associated polyamine content in Zea mays and Phaseolus vulgaris. Acta Physiol Plant 34(3):1145–1151
Lefevre I, Gratia E, Lutts S (2001) Discrimination between the ionic and osmotic components of salt stress in relation to free polyamine level in rice (Oryza sativa). Plant Sci 161(5):943–952
Lindner RC (1944) Rapid analytical method for some of the more inorganic constituents of plants tissue. Plant Physiol 19:76–89
Lingua G, Franchin C, Todeschini V, Castiglione S, Biondi S, Burlando B, Parravicini V, Torrigiani P, Berta G (2008) Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ Pollut 153(1):137–147
Liu JH, Inoue H, Moriguchi T (2008) Salt stress-mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environ Exp Bot 62(1):28–35
López-Gómez M, Cobos-Porras L, Hidalgo-Castellanos J, Lluch C (2014) Occurrence of polyamines in root nodules of Phaseolus vulgaris in symbiosis with Rhizobium tropici in response to salt stress. Phytochemistry 107:32–41
López-Gómez M, Hidalgo-Castellanos J, Muñoz-Sánchez JR, Marín-Peña AJ, Lluch C, Herrera-Cervera JA (2017) Polyamines contribute to salinity tolerance in the symbiosis Medicago truncatula-Sinorhizobium meliloti by preventing oxidative damage. Plant Physiol Biochem 116:9–17
Mehlich A (1953) Determination of P, ca, mg, K, Na and NH4. In: Short test methods used in soil testing division. Department of Agriculture, Raleigh
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Munns R, Wallace PA, Teakle NL, Colmer TD (2010) Measuring soluble ion concentrations (Na+, K+, cl−) in salt-treated plants. In: Sunkar R (ed) Plant stress tolerance. Methods in Molecular Biology. Springer, Berlin, pp 371–382
Ndoye F, Kane A, Diedhiou AG, Bakhoum N, Fall D, Sadio O, Sy MO, Noba K, Diouf D (2015) Effects of dual inoculation with arbuscular mycorrhizal fungi and rhizobia on Acacia senegal (L.) Willd. Seedling growth and soil enzyme activities in Senegal. Int J Biosci 6(2):36–48
Nelson DW, Sommers LE (1973) Determination of total nitrogen in plant material. Agron J 65(1):109–112
Nogales A, Aguirreolea J, Santa María E, Camprubí A, Calvet C (2009) Response of mycorrhizal grapevine to Armillaria mellea inoculation: disease development and polyamines. Plant Soil 317(1–2):177
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of soil analysis, Agron. No. 9, part 2- chemical and microbiological properties, 2nd edn. American Society Agronomy, Madison, pp 403–430
Oufdou K, Benidire L, Lyubenova L, Daoui K, Fatemi ZEA, Schröder P (2014) Enzymes of the glutathione–ascorbate cycle in leaves and roots of rhizobia-inoculated faba bean plants (Vicia faba L.) under salinity stress. Eur J Soil Biol 60:98–103
Peng J, Li Y, Shi P, Chen X, Lin H, Zhao B (2011) The differential behavior of arbuscular mycorrhizal fungi in interaction with Astragalus sinicus L. under salt stress. Mycorrhiza 21(1):27–33
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Porcel R, Aroca R, Azcon R, Ruiz-Lozano JM (2016) Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 26(7):673–684
Quinet M, Ndayiragije A, Lefevre I, Lambillotte B, Dupont-Gillain CC, Lutts S (2010) Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J Exp Bot 61(10):2719–2733
Ren CG, Bai YJ, Kong CC, Bian B, Xie ZH (2016) Synergistic interactions between salt-tolerant rhizobia and arbuscular mycorrhizal fungi on salinity tolerance of Sesbania cannabina plants. J Plant Growth Regul 35(4):1098–1107
Rohyadi A, Noviani R, Isnaini M (2017) Responses of cowpea genotypes to arbuscular mycorrhiza. Agrivita J Agric Sci 39(3):288–295
Sannazzaro AI, Echeverría M, Albertó EO, Ruiz OA, Menéndez AB (2007) Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol Biochem 45(1):39–46
Sa TM, Israel DW (1991) Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiol 97(3):928–935
Saxena KB, Nadarajan N (2010) Prospects of pigeonpea hybrids in Indian agriculture. Electron J Plant Breed 1(4):1107–1117
Schübler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Sengupta A, Chakraborty M, Saha J, Gupta B, Gupta K (2016) Polyamines: osmoprotectants in plant abiotic stress adaptation. In: Osmolytes and plants acclimation to changing environment: emerging omics technologies springer, New Delhi, pp 97–127
Shabala S, Cuin TA, Pottosin I (2007) Polyamines prevent NaCl induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett 581(10):1993–1999
Shi K, Huang YY, Xia XJ, Zhang YL, Zhou YH, Yu JQ (2008) Protective role of putrescine against salt stress is partially related to the improvement of water relation and nutritional imbalance in cucumber. J Plant Nutr 31(10):1820–1831
Talaat NB, Shawky BT (2013) Modulation of nutrient acquisition and polyamine pool in salt-stressed wheat (Triticum aestivum L.) plants inoculated with arbuscular mycorrhizal fungi. Acta Physiol Plant 35(8):2601–2610
Tejera NA, Campos R, Sanjuan J, Lluch C (2004) Nitrogenase and antioxidant enzyme activities in Phaseolus vulgaris nodules formed by Rhizobium tropici isogenic strains with varying tolerance to salt stress. J Plant Physiol 161(3):329
Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240(1):1–8
Upreti KK, Murti GSR (2010) Response of grape rootstocks to salinity: changes in root growth, polyamines and abscisic acid. Biol Plant 54(4):730–734
Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313(2):369–375
Vales MI, Rao GR, Sudini H, Patil SB, Murdock LL (2014) Effective and economic storage of pigeonpea seed in triple layer plastic bags. J Stored Prod Res 58:29–38
Varshney RK, Penmetsa RV, Dutta S, Kulwal PL, Saxena RK, Datta S, Sharma TR, Cook DR (2010) Pigeonpea genomics initiative (PGI): an international effort to improve crop productivity of pigeonpea (Cajanus cajan L.). Mol Breed 26(3):393–408
Vassileva V, Ignatov G (1999) Polyamine-induced changes in symbiotic parameters of the Galega orientalis–Rhizobium galegae nitrogen-fixing system. Plant Soil 210(1):83–91
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils: effects of variations in digestion conditions and of organic soil constituents. Soil Sci 63(4):251–264
Walters D (2003) Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phytol 159(1):109–115
Wilde P, Manal A, Stodden M, Sieverding E, Hildebrandt U, Bothe H (2009) Biodiversity of arbuscular mycorrhizal fungi in roots and soils of two salt marshes. Environ Microbiol 11(6):1548–1561
Wisniewski JP, Brewin NJ (2002) Possible Role for Diamine Oxidase in Infection Threads. In: Nitrogen Fixation: From Molecules to Crop Productivity Springer, Dordrecht. pp 259–259
Wisniewski JP, Rathbun EA, Knox JP, Brewin NJ (2000) Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol Plant-Microbe Interact 13(4):413–420
Wu H, Shabala L, Barry K, Zhou M, Shabala S (2013) Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiol Plant 149(4):515–527
Wu Q, Zou YN, He XH (2010) Exogenous putrescine, not spermine or spermidine, enhances root mycorrhizal development and plant growth of trifoliate orange (Poncirus trifoliata) seedlings. Int J Agric Biol 12:576–580
Wu QS, He XH, Zou YN, Liu CY, Xiao J, Li Y (2012) Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul 68(1):27–35
Xu Y, Shi GX, Ding CX, Xu XY (2011) Polyamine metabolism and physiological responses of Potamogeton crispus leaves under lead stress. Rus J Plant Physiol 58:460–466
Yasuta Y, Kokubun M (2014) Salinity tolerance of super-nodulating soybean genotype En-b0-1. Plant Prod Sci 17(1):32–40
Zeid IM (2004) Response of bean (Phaseolus vulgaris) to exogenous putrescine treatment under salinity stress. Pak J Biol Sci 7(2):219–225
Zhang GW, Xu SC, Hu QZ, Mao WH, Gong YM (2014) Putrescine plays a positive role in salt-tolerance mechanisms by reducing oxidative damage in roots of vegetable soybean. J Integr Agric 13:349–357
Zhang Y, Xie L, Xiong B, Zeng M, Liu J, Yu D, Yuan J (2003) Effect of polyamine on growth and development of arbuscular mycorrhizal fungi in vitro culture condition. Mycosystema 22(3):417–423
Zhao FG, Sun C, Liu YL, Zhang WH (2003) Relationship between polyamine metabolism in roots and salt tolerance of barley seedlings. Acta Bot Sin 45:295–300
Zwiazek JJ, Blake TJ (1991) Early detection of membrane injury in black spruce (Picea mariana). Can J For Res 21:401–404
Acknowledgments
We gratefully acknowledge the University Grants Commission and Department of Biotechnology, Government of India, for providing financial support in undertaking the research work. We are also thankful to TERI, New Delhi, and Pulse laboratory, IARI, New Delhi, for providing the biological research material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
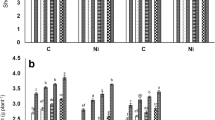
Effect of Putrescine (Put) and arbuscular mycorrhizal (AM, Rhizophagus irregularis) inoculation on a) potassium to sodium ratio (K+/Na+) and b) calcium to sodium ratio (Ca2+/Na+) in roots of differentially salt-tolerant Pusa 2001 and Pusa 991 pigeonpea genotypes under different levels of salinity stress (0–100 mM). Each value is the mean of six replicates ± standard error (SE). Different letters above each line indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. –Put–AM = Put unprimed non-AM plants; +Put–AM = Put primed non-AM plants; –Put+AM = Put unprimed AM plants; +Put+AM = Put primed AM plants (PNG 91 kb)
ESM 2
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Garg, N., Sharma, A. Role of putrescine (Put) in imparting salt tolerance through modulation of put metabolism, mycorrhizal and rhizobial symbioses in Cajanus cajan (L.) Millsp.. Symbiosis 79, 59–74 (2019). https://doi.org/10.1007/s13199-019-00621-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-019-00621-7