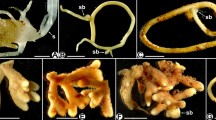
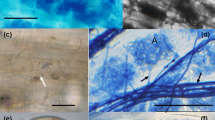
Abstract
Mycorrhiza is an important factor in plant morphogenesis, especially in root formation. It has been shown that mycorrhizal and non-mycorrhizal plants differ in root length and diameter; however, the underlying anatomical features have been poorly described. In the present work, we analysed functionally divergent roots of 28 species of dicotyledonous herbaceous plants (the Apiaceae, Asteraceae, Caryophyllaceae, Lamiaceae, and Polygonaceae families) from the Middle Urals, Russia. Based on our data and those of previous reports, we divided all species into three groups, non-mycorrhizal (6 species), mycorrhizal (9), and variable mycorrhizal (13), and compared general characteristics of their root systems and anatomical features of the finest roots. The root system of non-mycorrhizal plants was more branched compared to mycorrhizal, which possibly facilitates the uptake of water and mineral nutrients in the absence of fungal symbionts. The main difference was that the cortex of the mycorrhizal species’ roots was significantly thicker due to 4–6 cell layers while those of non-mycorrhizal had no more than 4 layers. Moreover, the cortex was apparently retained for a relatively long time. Analysis of variable mycorrhizal plants revealed intermediate values between two contrast groups. We suggest that a greater number of cortical cell layers in the finest roots and a prolonged retention of the cortex are intrinsic for mycorrhizal species; nevertheless, further research is needed to assess whether it is applicable to other dicotyledonous plant species.


Similar content being viewed by others
References
Akhmetzhanova AA, Soudzilovskaia NA, Onipchenko VG, Cornwell WK, Agafonov VA, Selivanov IA, Cornelissen JH (2012) A rediscovered treasure: mycorrhizal intensity database for 3000 vascular plant species across the former Soviet Union. Ecology 93:689–690. https://doi.org/10.1890/11-1749.1
Barker SJ, Tagu D, Delp G (1998) Regulation of root and fungal morphogenesis in mycorrhizal symbioses. Plant Physiol 116:1201–1207. https://doi.org/10.1104/pp.116.4.1201
Berntson GM (1997) Topological scaling and plant root system architecture: developmental and functional hierarchies. New Phytol 135:621–634. https://doi.org/10.1046/j.1469-8137.1997.00687.x
Berta G, Fusconi A, Trotta A, Scannerini S (1990) Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytol 114:207–215. https://doi.org/10.1111/j.1469-8137.1990.tb00392.x
Berta G, Sampo S, Gamalero E, Massa N, Lemanceau P (2005) Suppression of Rhizoctonia root-rot of tomato by Glomus mossae BEG12 and Pseudomonas fluorescens A6RI is associated with their effect on the pathogen growth and on the root morphogenesis. Eur J Plant Pathol 111:279–288. https://doi.org/10.1007/s10658-004-4585-7
Betekhtina AA, Veselkin DV (2011) Prevalence and intensity of mycorrhiza formation in herbaceous plants with different types of ecological strategies in the Middle Urals. Russ J Ecol 42:192–198. https://doi.org/10.1134/S1067413611030040
Brundrett MC (1991) Mycorrhizas in natural ecosystems. In: Advances in ecological research, Vol 21. Elsevier, pp 171-313. doi:https://doi.org/10.1016/S0065-2504(08)60099-9
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304. https://doi.org/10.1046/j.1469-8137.2002.00397.x
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Brundrett MC, Kendrick B (1988) The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can J Bot 66:1153–1173. https://doi.org/10.1139/b88-166
Citernesi AS, Vitagliano C, Giovannetti M (1998) Plant growth and root system morphology of Olea europaea L. rooted cuttings as influenced by arbuscular mycorrhizas. J Hortic Sci Biotechnol 73:647–654. https://doi.org/10.1080/14620316.1998.11511028
Cornelissen J, Aerts R, Cerabolini B, Werger M, Van Der Heijden M (2001) Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129:611–619. https://doi.org/10.1007/s004420100752
Cornelissen JHC et al (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. https://doi.org/10.1071/BT02124
Cosme M, Fernández I, Van der Heijden MG, Pieterse CM (2018) Non-mycorrhizal plants: the exceptions that prove the rule. Trends Plant Sci 23(7):577–587. https://doi.org/10.1016/j.tplants.2018.04.004
Eissenstat DM (1992) Costs and benefits of constructing roots of small diameter. J Plant Nutr 15:763–782. https://doi.org/10.1080/01904169209364361
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42. https://doi.org/10.1046/j.1469-8137.2000.00686.x
Enstone DE, Peterson CA, Hallgren SW (2001) Anatomy of seedling tap roots of loblolly pine (Pinus taeda L). Trees 15:98–111. https://doi.org/10.1007/s004680000079
Fusconi A (2013) Regulation of root morphogenesis in arbuscular mycorrhizae: what role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann Bot 113:19–33. https://doi.org/10.1093/aob/mct258
Gutjahr C, Casieri L, Paszkowski U (2009) Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol 182(4):829–837. https://doi.org/10.1111/j.1469-8137.2009.02839.x
Hempel S, Götzenberger L, Kühn I, Michalski SG, Rillig MC, Zobel M, Moora M (2013) Mycorrhizas in the Central European flora: relationships with plant life history traits and ecology. Ecology 94:1389–1399. https://doi.org/10.1890/12-1700.1
Hetrick BAD (1991) Mycorrhizas and root architecture. Experientia 47(4):355–362. https://doi.org/10.1007/Bf01972077
Hishi T (2007) Heterogeneity of individual roots within the fine root architecture: causal links between physiological and ecosystem functions. J For Res 12(2):126–133. https://doi.org/10.1007/s10310-006-0260-5
Kumar P, Hallgren SW, Enstone DE, Peterson CA (2007) Root anatomy of Pinus taeda L.: seasonal and environmental effects on development in seedlings. Trees 21:693. https://doi.org/10.1007/s00468-007-0162-y
Lehnert M, Krug M, Kessler M (2017) A review of symbiotic fungal endophytes in lycophytes and ferns - a global phylogenetic and ecological perspective. Symbiosis 71(2):77–89. https://doi.org/10.1007/s13199-016-0436-5
Li H, Liu B, McCormack ML, Ma Z, Guo D (2017) Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol 216:1140–1150. https://doi.org/10.1111/nph.14710
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195:823–831. https://doi.org/10.1111/j.1469-8137.2012.04198.x
McKenzie BE, Peterson CA (1995) Root browning in Pinus banksiana lamb. And Eucalyptus pilularis Sm. 1. Anatomy and permeability of the white and tannin zones. Botanica Acta 108:127–137. https://doi.org/10.1111/j.1438-8677.1995.tb00842.x
Olah B, Briere C, Becard G, Denarie J, Gough C (2005) Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J 44(2):195–207. https://doi.org/10.1111/j.1365-313X.2005.02522.x
Raven JA, Edwards D (2001) Roots: evolutionary origins and biogeochemical significance. J Exp Bot 52:381–401. https://doi.org/10.1093/jexbot/52.suppl_1.381
Salpagarova FS, Onipchenko VG, Agafonov VA, Adzhiev RK (2012) Specific root length of alpine plants in the Northwest Caucasus. Russia Bull Moscow Soc Nat 117:69–76 (in Russian)
Salpagarova FS, van Logtestijn RSP, Onipchenko VG, Akhmetzhanova AA, Agafonov VA (2014) Nitrogen content in fine roots and the structural and functional adaptations of alpine plants. Biol Bull Rev 4:243–251. https://doi.org/10.1134/S2079086414030074
Schellenbaum L, Berta G, Ravolanirina F, Tisserant B, Gianinazzi S, Fitter AH (1991) Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L). Ann Bot 68:135–141. https://doi.org/10.1093/oxfordjournals.aob.a088231
Selivanov IA (1981) Mycosymbiotrophism as form of consortive connections in the vegetation cover of the Soviet Union. Nauka, Moscow (in Russian)
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic press, London
St John TV (1980) Root size, root hairs and mycorrhizal infection: a re-examination of Baylis's hypothesis with tropical trees. New Phytol 84:483–487. https://doi.org/10.1111/j.1469-8137.1980.tb04555.x
Tester M, Smith S, Smith F (1987) The phenomenon of "nonmycorrhizal" plants. Can J Bot 65:419–431
Van der Heijden MGA, Cornelissen JHC (2002) The critical role of plant-microbe interactions on biodiversity and ecosystem functioning: arbuscular mycorrhizal associations as an example biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, London, pp 181–194. https://doi.org/10.1111/j.1061-2971.2004.120401.x
Veselkin DV, Betekhtina AA (2013) Testing the hypotheses about the difference between the sizes of the roots due to the type of ecological strategy and mycotrophic status of the plant species. Russia Bull Moscow Soc Nat 118:42–49 (in Russian)
Vigo C, Norman JR, Hooker JE (2000) Biocontrol of the pathogen Phytophthora parasitica by arbuscular mycorrhizal fungi is a consequence of effects on infection loci. Plant Pathol 49:509–514. https://doi.org/10.1046/j.1365-3059.2000.00473.x
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. https://doi.org/10.1007/s00572-005-0033-6
Wells CE, Eissenstat DM (2002) Beyond the roots of young seedlings: the influence of age and order on fine root physiology. J Plant Growth Regul 21:324–334. https://doi.org/10.1111/j.1438-8677.1995.tb00842.x
Acknowledgements
The assistance of Tatiana Rasskazova (UrFU, Foreign Languages Dpt.) and Ian Miller (UrFU, Linguistics) in the preparation of this article is acknowledged and appreciated. We would also like to thank two anonymous reviewers for their constructive comments. This project was partly supported by the Ministry of Education and Science of the Russian Federation within the State Task Programme (Project No. 6.7696.2017/8.9). The part of the project led by Dr. Denis Veselkin was performed under the State Contract of the Institute of Plant and Animal Ecology, UB RAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Betekhtina, A.A., Veselkin, D.V. Mycorrhizal and non-mycorrhizal dicotyledonous herbaceous plants differ in root anatomy: evidence from the Middle Urals, Russia. Symbiosis 77, 133–140 (2019). https://doi.org/10.1007/s13199-018-0571-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-018-0571-2