Abstract
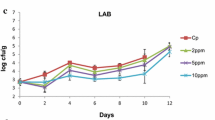
The aim of the present study was to investigate the combined effect of chitosan dip (1% w/v) and vacuum packaging on the shelf life of fresh chicken burgers packaged in LDPE/PA/LDPE bags and stored at 4 ± 1 °C for up to 12 days. Furthermore, the possible correlation among microbiological, physico-chemical and sensory indices was investigated. Burger treatments included: aerobic packaging (AP, control), vacuum packaging (VP), chitosan dipping (CHI), and vacuum packaging plus chitosan dipping (VP + CHI). Microbiological [Total viable count (TVC), Pseudomonas spp., Brochothrix thermosphacta, Enterobacteriaceae, Lactic acid bacteria (LAB)], physicochemical [color, pH, total volatile basic Nitrogen (TVB-N), and Thiobarbituric acid (TBA)] and sensory (odor, taste, and texture) analyses were carried out. Results showed that the majority of microbiological, physico-chemical, and sensory analysis parameters varied significantly (p < 0.05) depending on treatment. Based primarily on sensory, followed by microbiological and physico-chemical data, the shelf life of chicken burgers was 4 days for AP samples, 8 days for VP samples, 10 days for CHI treated samples, and 12 days for the VP + CHI treated samples. Finally, a positive and significant correlation (p < 0.05) was observed among most microbiological, sensory, and physico-chemical data, introducing new data relating initial TVC to TVB-N values regarding alternative treatments of minced chicken meat for its optimum preservation.




Similar content being viewed by others
Abbreviations
- LDPE/PA/LDPE:
-
Low density polyethylene/polyamide/low density polyethylene
- AP:
-
Aerobic packaging
- VP:
-
Vacuum packaging
- CHI:
-
Chitosan
- TVC:
-
Total viable count
- TVB-N:
-
Total volatile basic nitrogen
- TBA:
-
Thiobarbituric acid
- LAB:
-
Lactic acid Bacteria
- MAP:
-
Modified atmosphere packaging
- ANOVA:
-
Analysis of variance
References
Association of Official Chemists (AOAC) (2002) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Washington, DC
Bassi R, Prasher SO, Simpson BK (1999) Effects of organic acids on the adsorption of heavy metal ions by chitosan flakes. J Environ Sci Health 34:289–294. https://doi.org/10.1080/10934529909376836
Bordenave N, Grelier S, Coma V (2007) Water and moisture susceptibility of chitosan and paper-based materials: structure-property relationships. J Agric Food Chem 55(23):9479–9488. https://doi.org/10.1021/jf070595i
Brody AL (1997) Packaging of food. In: Brody AL, Marsh KS (eds) The Wiley encyclopedia of packaging, 2nd edn. Wiley, New York, pp 699–704
Byun JS, Min JS, Kim IS, Kim JW, Chung MS, Lee M (2003) Comparison of indicators of microbial quality of meat during aerobic cold storage. J Food Protect 66:3839–3843. https://doi.org/10.4315/0362-028X-66.9.1733
Castellano PH, Holzapfel WH, Vingolo GM (2004) The control of Listeria innocua and Lactobacillus sakei in broth and meat slurry with the bacteriocinogenic strain Lactobacillus casei CRL705. Food Microbiol 21:291–298. https://doi.org/10.1016/j.ijfoodmicro.2016.01.022
Chouliara E, Karatapanis A, Savvaidis IN, Kontominas MG (2007) Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4°C. Food Microbiol 24(6):607–617. https://doi.org/10.1016/j.fm.2006.12.005
Dias MR, Dianin KCS, Bersot LS, Nero LA (2017) Self-monitoring microbiological criteria for the assessment of hygienic procedures during chicken slaughtering. Braz J Poultry Sci 19(2):317–324. https://doi.org/10.1590/1806-9061-2016-0381
Economou T, Pournis N, Ntzimani A, Savvaidis IN (2009) Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem 114(4):1470–1476. https://doi.org/10.1016/j.foodchem.2008.11.036
European Food Safety Authority (EFSA) (2011) Scientific Opinion on the substantiation of health claims related to chitosan and reduction in body weight (ID 679, 1499), maintenance of normal blood LDL-cholesterol concentrations (ID 4663), reduction of intestinal transit time (ID 4664) and reduction of inflammation (ID 1985) pursuant to Article 13(1) of Regulation (EC) No 1924/20061EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) EFSA J 9(6):2214
Gänzle MG (2015) Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci 2:106–117. https://doi.org/10.1016/j.cofs.2015.03.001
Gertzou IN, Karabagias IK, Drosos PE, Riganakos KA (2017) Effect of combination of ozonation and vacuum packaging on shelf life extension of fresh chicken legs during storage under refrigeration. J Food Eng 213:18–26. https://doi.org/10.1016/j.jfoodeng.2017.06.026
Goy RC, Britto DD, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polímeros 19:241–247. https://doi.org/10.1590/S0104-14282009000300013
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int J Food Microbiol 71:235–244. https://doi.org/10.1016/s0168-1605(01)00609-2
Higueras L, López-Carballo G, Hernández-Muñoz P, Gavara R, Rollini M (2013) Development of a novel antimicrobial film based on chitosan with LAE (ethyl-Nα-dodecanoyl-L-arginate) and its application to fresh chicken. Int J Food Microbiol 165:339–345. https://doi.org/10.1016/j.ijfoodmicro.2013.06.003
Jääskeläinen E, Hultman J, Parshintsev J, Riekkola ML, Björkroth J (2016) Development of spoilage bacterial community and volatile compounds in chilled beef under vacuum or high oxygen atmospheres. Int J Food Microbiol 223:25–32. https://doi.org/10.1016/j.ijfoodmicro.2016.01.022
Jay JM, Loessner MJ, Golden DA (2005) Taxonomy role and significance of microorganisms in foods. Modern food microbiology, 7th edn. Springer Science and Business Media Inc., New York, pp. 13–31
Jimenez SM, Salsi MS, Tiburzi MC, Rafaghelli RC, Tessi MA, Coutaz VR (1997) Spoilage microflora in fresh chicken breast stored at 4°C: influence of packaging methods. J Appl Microbiol 83:613–618. https://doi.org/10.1046/j.1365-2672.1997.00276.x
Karam L, Roustom R, Abiad MG, El-Obeid T, Savvaidis IN (2019) Combined effects of thymol, carvacrol and packaging on the shelf-life of marinated chicken. Int J Food Microbiol 291:42–47. https://doi.org/10.1016/j.ijfoodmicro.2018.11.008
Kerch G, Korkhov V (2011) Effect of storage time and temperature on structure, mechanical and barrier properties of chitosan-based films. Eur Food Res Technol 232(1):17–22. https://doi.org/10.1007/s00217-010-1356-x
Kerry JP, O’Grady MN, Hogan SA (2006) Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: a review. Meat Sci 74:113–130. https://doi.org/10.1016/j.meatsci.2006.04.024
Khanjari A, Karabagias IK, Kontominas MG (2013) Combined effect of N, O carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT-Food Sci Technol 53:94–99. https://doi.org/10.1016/j.lwt.2013.02.012
Koutsoumanis KP, Taoukis P (2005) Meat safety, refrigerated storage and transport: modeling and management. In: Sofos JN (ed) Improving the safety of fresh meat. Woodhead Publishing, Elsevier, pp 503–561
Latou E, Mexis SF, Badeka AV, Kontakos S, Kontominas MG (2014) Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT-Food Sci Technol 55:263–268. https://doi.org/10.1016/j.lwt.2013.09.010
Mahdavi V, Hosseini SE, Sharifan A (2017) Effect of edible chitosan film enriched with anise (Pimpinella anisum L.) essential oil on shelf life and quality of the chicken burger. Food Sci Nutr 6(2):269–279. https://doi.org/10.1002/fsn3.544
McMillin KW (2017) Advancements in meat packaging. Meat Sci 132:153–162. https://doi.org/10.1016/j.meatsci.2017.04.015
Mexis SF, Chouliara E, Kontominas MG (2012) Shelf life extension of ground chicken meat using an oxygen absorber and a citrus extract. LWT-Food Sci Technol 49:21–27. https://doi.org/10.1016/j.lwt.2012.04.012
Mondry H (1996) Packaging systems for processed meat. In: Taylor SA, Raimundo M, Severini F, Smulders JM (eds) Meat quality and meat packaging. ECCEAMST, Utrecht, pp 323–333
Murphy RY, Marks BP (2000) Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties. Poultry Sci 79:99–104. https://doi.org/10.1093/ps/79.1.99
Pandey A, Joshi VK, Nigam PR, Soccol C (1999) Enterobacteriaceae, Coliforms and E. coli. In: Richard RK, Batt CA, Patel PD (eds) Encyclopedia of microbiology. American Press, New York, pp 604–610. https://doi.org/10.1016/j.fm.2016.05.007
Paparella A, Mazzarrino G, Chaves-Lopez C, Rossi C, Sacchetti G, Guerrieri O, Serio A (2016) Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol 59:23–31. https://doi.org/10.1016/j.fm.2016.05.007
Pavelková A, Kačániová M, Horská E, Rovná K, Hleba L, Petrová J (2014) The effect of vacuum packaging, EDTA, oregano and thyme oils on the microbiological quality of chicken’s breast. Anaerobe 29:128–133. https://doi.org/10.1016/j.anaerobe.2013.09.002
Petrou S, Tsiraki M, Giatrakou V, Savvaidis IN (2012) Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int J Food Microbiol 156(3):264–271. https://doi.org/10.1016/j.ijfoodmicro.2012.04.002
Pin C, Garcia de Fernando G, Ordonez JA (2002) Effect of modified atmosphere composition on the metabolism of glucose by Brochothrix thermosphacta. Appl Environ Microbiol 68:4441–4447. https://doi.org/10.1128/AEM.68.9.4441-4447.2002
Raafat D, von Bargen K, Haas A, Sahl HG (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74:3764–3773. https://doi.org/10.1128/AEM.00453-08
Remenant B, Jaffrès E, Dousset X, Pilet MF, Zagorec M (2015) Bacterial spoilers of food: behavior, fitness and functional properties. Food Microbiol 45:45–53. https://doi.org/10.1016/j.fm.2014.03.009
Sagoo S, Board R, Roller S (2002) Chitosan inhibits growth of spoilage microorganisms in chilled pork products. Food Microbiol 19:175–182. https://doi.org/10.1006/fmic.2001.0474
Severino R, Ferrari G, Vu KD, Donsì F, Salmieri S, Lacroix M (2015) Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils: modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella typhimurium on green beans. Food Control 50:215–222. https://doi.org/10.1016/j.foodcont.2014.08.029
Sirocchi V, Devlieghere F, Peelman N, Sagratini G, Maggi F, Vittori S, Ragaert P (2017) Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem 221:1069–1076. https://doi.org/10.1016/j.foodchem.2016.11.054
Šuput DZ, Lazić VL, Popović SZ, Hromiš NM (2015) Edible films and coatings: sources, properties and application. Food Feed Res 42(1):11–22. https://doi.org/10.5937/FFR1501011S
Van Wezemael L, Useland O, Verbeke W (2011) European consumer response to packaging technologies for improved beef safety. Meat Sci 89:45–51. https://doi.org/10.1016/j.meatsci.2011.03.019
Yen MO, Yang JH, Mau JL (2008) Antioxidant properties of chitosan from crab shells. Carbohydr Polym 74:840–844. https://doi.org/10.1016/j.carbpol.2008.05.003
Acknowledgements
The authors are grateful to the Agricultural Poultry Cooperative of Pindos Ioannina (Pindos S.A., Ioannina, Greece) for the donation of chicken fillets.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Assanti, E., Karabagias, V.K., Karabagias, I.K. et al. Shelf life evaluation of fresh chicken burgers based on the combination of chitosan dip and vacuum packaging under refrigerated storage. J Food Sci Technol 58, 870–883 (2021). https://doi.org/10.1007/s13197-020-04601-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04601-4