Abstract
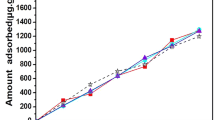
Succinic acid and l-malic acid are known to affect calcite dissolution/crystallization due to their tendency to adsorb on calcite’s surface. Taking into account the structural similarity among succinic acid, l-malic acid, and d-malic acid, a comparative study of their adsorption behavior on calcite was investigated for clarifying their adsorption mechanism. Kinetic results showed that l-malic acid adsorption reached equilibrium within 12 h, while succinic acid required 48 h. Results from the pH effect study showed that the strength of surface complex of succinic acid on calcite was weaker, even compared to its aqueous complex with Ca2+ ion. Alternately, l-malic acid exhibited a much stronger surface complex strength, even compared to its aqueous complex with Ca2+ ion. The adsorption isotherm results indicated that the saturated adsorption amount of l-malic acid was twice that of succinic acid, suggesting the surface complex of succinic acid may occupy a larger space on calcite surface compared to that of l-malic acid. Based on these results and those from previous studies, we proposed that succinic acid adsorption is through its two carboxyl groups binding to two adjacent surface sites (> Ca+), and not only one. Also, l-malic acid adsorption occurs through one carboxyl group and one hydroxyl, which forms a “claw” on calcite surface. The similar adsorption behavior between l-malic acid and d-malic acid reinforces this proposition for l-malic acid adsorption mechanism. Our study will serve as good reference information to provide better understanding into the adsorption of other carboxylic acids on calcite.




Similar content being viewed by others
References
Amrhein C, Jurinak JJ, Moore WM (1985) Kinetics of calcite dissolution as affected by carbon dioxide partial pressure. Soil Sci Soc Am J 49:1393–1398
Compton RG, Brown CA (1995) The inhibition of calcite dissolution/precipitation: 1, 2-dicarboxylic acids. J Colloid Interface Sci 170:586–590
Ehrlich H, Koutsoukos PG, Demadis KD, Pokrovsky OS (2009) Principles of demineralization: modern strategies for the isolation of organic frameworks. Micron 40:169–193
Einax M, Richter T, Nimmrich M, Rahe P, Stara IG, Stary I, Kuhnle A, Maass P (2016) Growth kinetics of racemic heptahelicene-2-carboxylic acid nanowires on calcite (104). J Chem Phys 145:134702
Feininger T (2013) An introduction to the rock-forming minerals. Can Miner 51:663–664
Geffroy C, Foissy A, Persello J, Cabane B (1999) Surface complexation of calcite by carboxylates in water. J Colloid Interface Sci 211:45–53
Hauke CM, Rahe P, Nimmrich M, Schütte J, Kittelmann M, Stara IG, Stary I, Rybacek J, Kuehnle A (2012) Molecular self-assembly of enantiopure heptahelicene-2-carboxylic acid on calcite (1014). J Phys Chem C 116:4637–4641
Heberling F, Trainor TP, Lützenkirchen J, Eng P, Denecke MA, Bosbach D (2011) Structure and reactivity of the calcite–water interface. J Colloid Interface Sci 354:843–857
Huang W, Ma R, Yang D, Liu X, Song J (2014) Organic acids secreted from plant roots under soil stress and their effects on ecological adaptability of plants. Agric Sci Technol 15:1167–1173
Kharaka YK, Thordsen JJ, Hovorka SD, Nance HS, Cole DR, Phelps TJ, Knauss KG (2009) Potential environmental issues of CO2 storage in deep saline aquifers: geochemical results from the Frio-I brine pilot test, Texas, USA. Appl Geochem 24:1106–1112
Kpomblekou-A K, Tabatabai MA (2003) Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agric Ecosyst Environ 100:275–284
Li Q, Wang D, Wu Y, Li W, Zhang Y, Xing J, Su Z (2010) One step recovery of succinic acid from fermentation broth by crystallization. Sep Purif Technol 72:294–300
Li Z, Huang L, Chen Y, Miao Y, Liu D, Xu Z (2015) Sorption of malonate onto calcite in open-system. Environ Chem 34:1940–1947 (in Chinese)
Marschner H (1995) Mineral nutrition in higher plants. Academic, London
Martinez RE, Gardés E, Pokrovsky OS, Schott J, Oelkers EH (2010) Do photosynthetic bacteria have a protective mechanism against carbonate precipitation at their surface. Geochimica et Coschimica Acta 74:1329–1337
Meldrum FC, Hyde ST (2001) Morphological influence of magnesium and organic additives on the precipitation of calcite. J Cryst Growth 231:544–558
Michard G (2002) Chimie des eaux naturelles. Publisud, France
Montanari G, Lakshtanov LZ, Tobler DJ, Dideriksen K, Dalby KN, Bovet N, Stipp SLS (2016) Effect of aspartic acid and glycine on calcite growth. Cryst Growth Des 16:4813–4821
Muryanto S, Bayuseno AP, Ma’mun H, Usamah M (2014) Calcium carbonate scale formation in pipes: effect of flow rates, temperature, and malic acid as additives on the mass and morphology of the scale. Proc Chem 9:69–76
Oelkers EH, Schott J (2005) Geochemical aspects of CO2 sequestration. Chem Geol 217:183–186
Oelkers EH, Golubev SV, Pokrovsky OS, Bénézeth P (2011) Do organic ligands affect calcite dissolution rates. Geochim Cosmochim Acta 75:1799–1813
Okhrimenko DV, Nissenbaum J, Andersson MP, Olsson MHM, Stipp SLS (2013) Energies of the adsorption of functional groups to calcium carbonate polymorphs: the Importance of −OH and −COOH groups. Langmuir 29:11062–11073
Pokrovsky OS, Schott J (2002) Surface chemistry and dissolution kinetics of divalent metal carbonates. Environ Sci Technol 36:426–432
Pokrovsky OS, Golubev SV, Jordan G (2009) Effect of organic and inorganic ligands on calcite and magnesite dissolution rates at 60 °C and 30 atm pCO2. Chem Geol 265:33–43
Salinas-Nolasco MF, Méndez-Vivar J, Lara VH, Bosch P (2004) Passivation of the calcite surface with malonate ion. J Colloid Interface Sci 274:16–24
Strathmann TJ, Myneni SCB (2004) Speciation of aqueous Ni(II)-carboxylate and Ni(II)-fulvic acid solutions: combined ATR-FTIR and XAFS analysis. Geochim Cosmochim Acta 68:3441–3458
Teng H, Chen Y, Pauli E (2006) Direction specific interactions of 1, 4-dicarboxylic acid with calcite surfaces. J Am Chem Soc 128:14482–14484
Van Cappellen P, Charlet L, Stumm W, Wersin P (1993) A surface complexation model of the carbonate mineral-aqueous solution interface. Geochim Cosmochim Acta 57:3505–3518
Vargas E, Ruiz MA, Ferrero FJ, Campuzano S, Ruiz-Valdepeñas Montiel V, Reviejo AJ, Pingarrón JM (2016) Automatic bionalyzer using an integrated amperometric biosensor for the determination of l-malic acid in wines. Talanta 158:6–13
Wada N, Yamashita K, Umegaki T (1999) Effects of carboxylic acids on calcite formation in the presence of Mg2+ ions. J Colloid Interface Sci 212:357–364
Wada N, Kanamura K, Umegaki T (2001) Effects of carboxylic acids on the crystallization of calcium carbonate. J Colloid Interface Sci 233:65–72
Xie A, Shen Y, Zhang C, Yuan Z, Zhu X, Yang Y (2005) Crystal growth of calcium carbonate with various morphologies in different amino acid systems. J Cryst Growth 285:436–443
Zuo M, Renman G, Gustafsson JP, Renman A (2015) Phosphorus removal performance and speciation in virgin and modified argon oxygen decarburization slag designed for wastewater. Water Res 87:271–281
Acknowledgements
This work is financially supported by the Natural Science Foundation of China (nos. 41303096, 41201515) and Major Science and Technology Program for Water Pollution Control and Treatment (2015ZX07204-002).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Xiao, J., Huang, L. et al. Comparative study of carboxylic acid adsorption on calcite: l-malic acid, d-malic acid and succinic acid. Carbonates Evaporites 34, 1131–1139 (2019). https://doi.org/10.1007/s13146-017-0416-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-017-0416-8