Abstract
Purpose
Oxidized low-density lipoprotein (oxLDL) plays a key role in endothelial dysfunction, vascular inflammation, and atherogenesis. The aim of this study was to assess blood clearance and in vivo kinetics of radiolabeled oxLDL in mice.
Methods
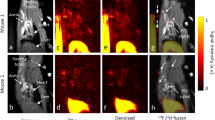
We synthesized 123I–oxLDL by the iodine monochloride method, and performed an uptake study in CHO cells transfected with lectin-like oxLDL receptor-1 (LOX-1). In addition, we evaluated the consistency between the 123I–oxLDL autoradiogram and the fluorescence image of DiI-oxLDL after intravenous injection for both spleen and liver. Whole-body dynamic planar images were acquired 10 min post injection of 123I–oxLDL to generate regional time-activity curves (TACs) of the liver, heart, lungs, kidney, head, and abdomen. Regional radioactivity for those excised tissues as well as the bladder, stomach, gut, and thyroid were assessed using a gamma counter, yielding percent injected dose (%ID) and dose uptake ratio (DUR). The presence of 123I–oxLDL in serum was assessed by radio-HPLC.
Results
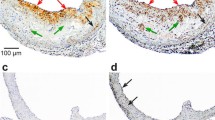
The cellular uptakes of 123I–oxLDL were identical to those of DiI-oxLDL, and autoradiograms and fluorescence images also exhibited consistent distributions. TACs after injection of 123I–oxLDL demonstrated extremely fast kinetics. The radioactivity uptake at 10 min post-injection was highest in the liver (40.8 ± 2.4% ID). Notably, radioactivity uptake was equivalent throughout the rest of the body (39.4 ± 2.7% ID). HPLC analysis revealed no remaining 123I–oxLDL or its metabolites in the blood.
Conclusion
123I–OxLDL was widely distributed not only in the liver, but also throughout the whole body, providing insight into the pathophysiological effects of oxLDL.






Similar content being viewed by others
References
Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–44.
Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104(4):503–16.
Kopprasch S, Pietzsch J, Ansurudeen I, et al. Prediabetic and diabetic in vivo modification of circulating low-density lipoprotein attenuates its stimulatory effect on adrenal aldosterone and cortisol secretion. J Endocrinol. 2009;200(1):45–52.
Portelinha A, Belo L, Cerdeira AS, et al. Lipid levels including oxidized LDL in women with history of preeclampsia. Hypertens Pregnancy. 2010;29(1):93–100.
Dai L, Zhang Z, Winyard PG, et al. A modified form of low-density lipoprotein with increased electronegative charge is present in rheumatoid arthritis synovial fluid. Free Radic Biol Med. 1997;22(4):705–10.
Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112(5):651–7.
Inoue N, Okamura T, Kokubo Y, et al. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56(4):550–8.
Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299(19):2287–93.
Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266(4):2282–9.
Kamps JA, Kruijt JK, Kuiper J, van Berkel TJ. Characterization of the interaction of acetylated LDL and oxidatively modified LDL with human liver parenchymal and Kupffer cells in culture. Arterioscler Thromb. 1992;12(9):1079–87.
Ueda Y, Arai H, Kawashima A, et al. Different expression of modified low density lipoprotein receptors in rabbit peritoneal macrophages and Kupffer cells. Atherosclerosis. 1993;101(1):25–35.
Ling W, Lougheed M, Suzuki H, Buchan A, Kodama T, Steinbrecher UP. Oxidized or acetylated low density lipoproteins are rapidly cleared by the liver in mice with disruption of the scavenger receptor class a type I/II gene. J Clin Invest. 1997;100(2):244–52.
Steinbrecher UP, Witztum JL, Parthasarathy S, Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987;7(2):135–43.
Iuliano L, Signore A, Vallabajosula S, et al. Preparation and biodistribution of 99m technetium labelled oxidized LDL in man. Atherosclerosis. 1996;126(1):131–41.
Iida H, Nakagawara J, Hayashida K, et al. Multicenter evaluation of a standardized protocol for rest and acetazolamide cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine. J Nucl Med. 2010;51(10):1624–31.
Iida H, Narita Y, Kado H, et al. Effects of scatter and attenuation correction on quantitative assessment of regional cerebral blood flow with SPECT. J Nucl Med. 1998;39(1):181–9.
Fujita Y, Kakino A, Nishimichi N, et al. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin Chem. 2009;55(2):285–94.
Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–7.
Atsma DE, Kempen HJ, Nieuwenhuizen W, van’t Hooft FM. Pauwels EK. Partial characterization of low density lipoprotein preparations isolated from fresh and frozen plasma after radiolabeling by seven different methods. J Lipid Res. 1991;32(1):173–81.
Gullapalli RR, Demirel MC, Butler PJ. Molecular dynamics simulations of DiI-C18(3) in a DPPC lipid bilayer. Phys Chem Chem Phys. 2008;10(24):3548–60.
Yamamoto A, Sato H, Enmi J, et al. Use of a clinical MRI scanner for preclinical research on rats. Radiol Phys Technol. 2009;2(1):13–21.
Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82.
Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2(3):131–7.
Diehl KH, Hull R, Morton D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21(1):15–23.
R Core Team. R: A language and environment for statistical computing, version 3.2.2. R Foundation for Statistical Computing, Vienna, Austria; 2014. http://www.R-project.org/.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Sobal G, Resch U, Sinzinger H. Modification of low-density lipoprotein by different radioiodination methods. Nucl Med Biol. 2004;31(3):381–8.
Pietzsch J, Bergmann R, Wuest F, Pawelke B, Hultsch C, van den Hoff J. Catabolism of native and oxidized low density lipoproteins: in vivo insights from small animal positron emission tomography studies. Amino Acids. 2005;29(4):389–404.
Lougheed M, Moore ED, Scriven DR, Steinbrecher UP. Uptake of oxidized LDL by macrophages differs from that of acetyl LDL and leads to expansion of an acidic endolysosomal compartment. Arterioscler Thromb Vasc Biol. 1999;19(8):1881–90.
Nakano A, Inoue N, Sato Y, et al. LOX-1 mediates vascular lipid retention under hypertensive state. J Hypertens. 2010;28(6):1273–80.
Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–16.
Kreissl MC, Wu HM, Stout DB, et al. Noninvasive measurement of cardiovascular function in mice with high-temporal-resolution small-animal PET. J Nucl Med. 2006;47(6):974–80.
Palanisamy GS, Kirk NM, Ackart DF, et al. Uptake and accumulation of oxidized low-density lipoprotein during mycobacterium tuberculosis infection in guinea pigs. PLoS One. 2012;7(3):e34148.
Luoto P, Laitinen I, Suilamo S, Nagren K, Roivainen A. Human dosimetry of carbon-11 labeled N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide extrapolated from whole-body distribution kinetics and radiometabolism in rats. Mol Imaging Biol. 2010;12(4):435–42.
Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998;54(7):628–40.
Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182(1):1–15.
Acknowledgements
The authors would like to thank Dr. Kyoko Shioya, DVM from Laboratory of Animal Experiment and Medicine Management, National Cerebral and Cardiovascular Center, Osaka, Japan, for her assistance and advise on animal care and experimental procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Atushi Nakano, Hidekazu Kawashima, Yoshinori Miyake, Tsutomu Zeniya, Kazuhiro Koshino, Takashi Temma, Tetsuya Fukuda, Yoshiko Fujita, Akemi Kakino, Shigehiko Kanaya, and Tatsuya Sawamura declare that they have no conflict of interest. Hidehiro Iida received research grants from Chugai Yakuhin, Japan, Nihon Medi Physics, Japan and Molecular Imaging Labo, Japan. Akihide Yamamoto is paid by Molecular Imaging lab, Japan. This study was supported by the Budget for Nuclear Research of MEXT (Ministry of Education, Culture, Sports, Science and Technology Japan), a Grant for Translational Research from MHLW (Ministry of Health, Labor and Welfare, Japan), a Grant for Strategic Japanese-Finnish Research Cooperative Program on “Application of Medical ICT Devices” from Japan Agency for Medical Research and Development (AMED), Japan, and JSPS KAKENHI Grants (Number: 24,601,021 and 15 K01309).
Ethical Approval
The animal experiments in this study were conducted in accordance with guidelines for animal research on Human Care and Use of Laboratory Animals (Rockville, National Institute of Health/Office for Protection from Research Risks, 1996). The study protocol was approved by the Sub-committee for Laboratory Animal Welfare, National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan.
Informed Consent
The present study included only animal data, thus our institute approved that the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Nakano, A., Kawashima, H., Miyake, Y. et al. 123I–Labeled oxLDL Is Widely Distributed Throughout the Whole Body in Mice. Nucl Med Mol Imaging 52, 144–153 (2018). https://doi.org/10.1007/s13139-017-0497-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0497-2