Abstract
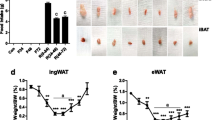
Some researchers have proposed important variations in adipose tissue among different strains of rats and mice in response to a high-caloric (hc) diet, but data concerning the mechanisms underlying these differences are scarce. The aim of the present research was to characterize different aspects of triacylglycerol (TG) metabolism and clock genes between Sprague-Dawley and Wistar rats. For this purpose, 16 male Sprague-Dawley and 16 male Wistar rats were divided into four experimental groups (n = 8) and fed either a normal-caloric (nc) diet or a hc diet for 6 weeks. After sacrifice, liver and epididymal, perirenal, mesenteric, and subcutaneous adipose tissue depots were dissected, weighed and immediately frozen. Liver TG content was quantified, RNA extracted for gene expression analysis and fatty acid synthase enzyme activity measured. Two-way ANOVA and Student’s t test were used to perform the statistical analyses. Under hc feeding conditions, Wistar rats were more prone to fat accumulation in adipose tissue, especially in the epididymal fat depot, due to their increased lipogenesis and fatty acid uptake. By contrast, both strains of rats showed similarly fatty livers after hc feeding. Peripheral clock machinery seems to be a potential explanatory mechanism for Wistar and Sprague-Dawley strain differences. In conclusion, Wistar strain seems to be the best choice as animal model in dietary-induced obesity studies.




Similar content being viewed by others
References
Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A (2005) Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 146:5631–5636
Barnea M, Madar Z, Froy O (2010) High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue. Obesity 18:230–238
Benoit B, Plaisancié P, Awada M, Géloën A, Estienne M, Capel F, Malpuech-Brugère C, Debard C, Pesenti S, Morio B, Vidal H, Rieusset J, Michalski MC (2013) High-fat diet action on adiposity, inflammation, and insulin sensitivity depends on the control low-fat diet. Nutr Res 33:952–960
Buettner R, Schölmerich J, Bollheimer LC (2007) High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity 15:798–808
Doi M (2012) Circadian clock-deficient mice as a tool for exploring disease etiology. Biol Pharm Bull 35:1385–1391
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Gajda A.M. PMA, Ricci M.R., Ulman E.A. (2007) Diet-induced metabolic syndrome in rodents model. In:Vincon Publishing Inc., Animal Lab News
Galan X, Llobera M, Ramírez I (1994) Lipoprotein lipase and hepatic lipase in Wistar and Sprague-Dawley rat tissues. Differences in the effects of gender and fasting. Lipids 29:333–336
Gómez-Zorita S, Fernández-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP (2013) Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition 29:1374–1380
Honma K, Hikosaka M, Mochizuki K, Goda T (2016) Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Metabolism 65:482–491
Kanasaki K, Koya D (2011) Biology of obesity: lessons from animal models of obesity. J Biomed Biotechnol 2011:197636
Kasaoka S, Yamagishi H, Kitano T (1999) Differences in the effect of iron-deficient diet on tissue weight, hemoglobin concentration and serum triglycerides in Fischer-344, Sprague-Dawley and Wistar rats. J Nutr Sci Vitaminol 45:359–366
Kiehn JT, Tsang AH, Heyde I, Leinweber B, Kolbe I, Leliavski A, Oster H (2017) Circadian rhythms in adipose tissue physiology. Compr Physiol 7:383–427
Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–421
Kučera O, Garnol T, Lotková H, Staňková P, Mazurová Y, Hroch M, Bolehovská R, Roušar T, Červinková Z (2011) The effect of rat strain, diet composition and feeding period on the development of a nutritional model of non-alcoholic fatty liver disease in rats. Physiol Res 60:317–328
Lasa A, Churruca I, Simón E, Fernández-Quintela A, Rodríguez V, Portillo M (2008) Trans-10, cis-12-conjugated linoleic acid does not increase body fat loss induced by energy restriction. Br J Nutr 100:1245–1250
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Malik VS, Willett WC, Hu FB (2013) Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 9:13–27
Mammi C, Calanchini M, Antelmi A, Cinti F, Rosano GM, Lenzi A, Caprio M, Fabbri A (2012) Androgens and adipose tissue in males: a complex and reciprocal interplay. Int J Endocrinol 789653:1–8
Marques C, Meireles M, Norberto S, Leite J, Freitas J, Pestana D, Faria A, Calhau C (2016) High-fat diet-induced obesity rat model: a comparison between Wistar and Sprague-Dawley rat. Adipocyte 5:11–21
McCarty (2001) Modulation of adipocyte lipoprotein lipase expression as a strategy for preventing or treating visceral obesity. Med Hypotheses 57:192–200
Miranda J, Churruca I, Fernández-Quintela A, Rodríguez VM, Macarulla MT, Simón E, Portillo MP (2009) Weak effect of trans-10, cis-12-conjugated linoleic acid on body fat accumulation in adult hamsters. Br J Nutr 102:1583–1589
Miranda J, Portillo MP, Madrid JA, Arias N, Macarulla MT, Garaulet M (2013) Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br J Nutr 110:1421–1428
Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N (2013) Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56:1129–1139
Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M (2012) Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin 33:173–181
Pallister T, Spector TD (2016) Food: a new form of personalised (gut microbiome) medicine for chronic diseases? J R Soc Med 109:331–336
Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S (2013) High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci 37:1350–1356
Postic C, Girard J (2008) The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab 34:643–648
Reno C, Fehn R (2006) High-fat diet induced insulin resistance is more robust and reliable in Wistar than Sprague-Dawley rats. In: Experimental Biology. San Francisco, p A A2096
Rosenstengel S, Stoeppeler S, Bahde R, Spiegel HU, Palmes D (2011) Type of steatosis influences microcirculation and fibrogenesis in different rat strains. J Investig Surg 24:273–282
Rosini TC, Silva AS, Moraes C (2012) Diet-induced obesity: rodent model for the study of obesity-related disorders. Rev Assoc Med Bras 58:383–387
Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH (2013) Circadian disruption leads to insulin resistance and obesity. Curr Biol 23:372–381
Soulage C, Zarrouki B, Soares AF, Lagarde M, Geloen A (2008) Lou/C obesity-resistant rat exhibits hyperactivity, hypermetabolism, alterations in white adipose tissue cellularity, and lipid tissue profiles. Endocrinology 149:615–625
Stöppeler S, Palmes D, Fehr M, Hölzen JP, Zibert A, Siaj R, Schmidt HH, Spiegel HU, Bahde R (2013) Gender and strain-specific differences in the development of steatosis in rats. Lab Anim 47:43–52
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308:1043–1045
Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A (2006) High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int 23:905–914
Yoshida C, Shikata N, Seki S, Koyama N, Noguchi Y (2012) Early nocturnal meal skipping alters the peripheral clock and increases lipogenesis in mice. Nutr Metab 9:78
Acknowledgements
The technical assistance of Asier Leniz in RNA isolation and quality assessment is gratefully acknowledged.
Funding
This study was supported by grants from the Instituto de Salud Carlos III (CIBERObn), Government of the Basque Country (IT-572-13) and University of the Basque Country (UPV/EHU) (ELDUNANOTEK UFI11/32). Itziar Eseberri is a recipient of a doctoral fellowship from the University of the Basque Country. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All animal experimental protocols were reviewed and approved by the ethics committee on animal welfare of our institution (Comité Ético de Experimentación Animal de la Universidad del País Vasco, CEEA-UPV/EHU).
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Miranda, J., Eseberri, I., Lasa, A. et al. Lipid metabolism in adipose tissue and liver from diet-induced obese rats: a comparison between Wistar and Sprague-Dawley strains. J Physiol Biochem 74, 655–666 (2018). https://doi.org/10.1007/s13105-018-0654-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0654-9