Abstract
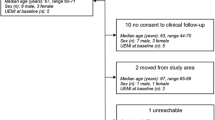
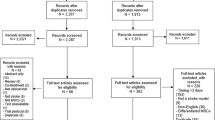
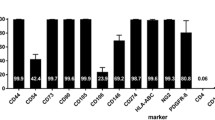
While preclinical stroke studies have shown that mesenchymal stem cells (MSCs) promote recovery, few randomized controlled trials (RCT) have assessed cell therapy in humans. In this RCT, we assessed the safety, feasibility, and efficacy of intravenous autologous bone marrow-derived MSCs in subacute stroke. ISIS-HERMES was a single-center, open-label RCT, with a 2-year follow-up. We enrolled patients aged 18–70 years less than 2 weeks following moderate-severe ischemic carotid stroke. Patients were randomized 2:1 to receive intravenous MSCs or not. Primary outcomes assessed feasibility and safety. Secondary outcomes assessed global and motor recovery. Passive wrist movement functional MRI (fMRI) activity in primary motor cortex (MI) was employed as a motor recovery biomarker. We compared “treated” and “control” groups using as-treated analyses. Of 31 enrolled patients, 16 patients received MSCs. Treatment feasibility was 80%, and there were 10 and 16 adverse events in treated patients, and 12 and 24 in controls at 6-month and 2-year follow-up, respectively. Using mixed modeling analyses, we observed no treatment effects on the Barthel Index, NIHSS, and modified-Rankin scores, but significant improvements in motor-NIHSS (p = 0.004), motor-Fugl-Meyer scores (p = 0.028), and task-related fMRI activity in MI-4a (p = 0.031) and MI-4p (p = 0.002). Intravenous autologous MSC treatment following stroke was safe and feasible. Motor performance and task-related MI activity results suggest that MSCs improve motor recovery through sensorimotor neuroplasticity.
ClinicalTrials.gov Identifier NCT 00875654.





Similar content being viewed by others
References
Sarraj A, Grotta JC. Stroke: new horizons in treatment. Lancet Neurol. 2014;13(1):2–3.
Moisan A, Favre I, Rome C, De Fraipont F, Grillon E, Coquery N, et al. Intravenous injection of clinical grade human MSCs after experimental stroke: functional benefit and microvascular effect. Cell Transplant. 2016;25(12):2157–71.
Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018:271678X18776802.
Dhere T, Copland I, Garcia M, Chiang KY, Chinnadurai R, Prasad M, et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease—a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44(5):471–81.
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59(12):1662–9.
Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE 2nd, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376(11):1047–53.
Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev. 2015;11(2):280–7.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45.
Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–82.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360–8.
Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43(8):2242–4.
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106.
Detante O, Moisan A, Hommel M, Jaillard A. Controlled clinical trials of cell therapy in stroke: meta-analysis at six months after treatment. Int J Stroke. 2017;12(7):748–51.
Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol. 2011;69(2):375–88.
Ramsey LE, Siegel JS, Baldassarre A, Metcalf NV, Zinn K, Shulman GL, et al. Normalization of network connectivity in hemispatial neglect recovery. Ann Neurol. 2016;80(1):127–41.
Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, et al. Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007;17(12):2980–7.
Richards LG, Stewart KC, Woodbury ML, Senesac C, Cauraugh JH. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia. 2008;46(1):3–11.
Favre I, Zeffiro TA, Detante O, Krainik A, Hommel M, Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke. 2014;45(4):1077–83.
Hannanu FF, Zeffiro TA, Lamalle L, Heck O, Renard F, Thuriot A, et al. Parietal operculum and motor cortex activities predict motor recovery in moderate to severe stroke. NeuroImage Clin. 2017;14:518–29.
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174(1):11–20.
Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10(1):31–40.
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–86.
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Med J. 1965;14:61–5.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7.
Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42(2):427–32.
Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):123–30.
Loubinoux I. Can fMRI measures of brain motor activation add significantly to other variables in the prediction of treatment response? Stroke. 2007;38(7):2032–3.
Mahdavi A, Azar R, Shoar MH, Hooshmand S, Mahdavi A, Kharrazi HH. Functional MRI in clinical practice: assessment of language and motor for pre-surgical planning. Neuroradiol J. 2015;28(5):468–73.
Savitz SI, Cramer SC, Wechsler L, Consortium S. Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke. 2014;45(2):634–9.
Choudhri AF, Patel RM, Siddiqui A, Whitehead MT, Wheless JW. Cortical activation through passive-motion functional MRI. AJNR Am J Neuroradiol. 2015;36(9):1675–81.
Blatow M, Reinhardt J, Riffel K, Nennig E, Wengenroth M, Stippich C. Clinical functional MRI of sensorimotor cortex using passive motor and sensory stimulation at 3 Tesla. J Magn Reson Imaging. 2011;34(2):429–37.
Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, et al. Brain representation of active and passive movements. NeuroImage. 1996;4(2):105–10.
Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001;21(5):592–607.
Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23(3):827–39.
Wilkinson L, Task Force on Statistical Inference. Statistical methods in psychology journals: guidelines and explanations. Am Psychol. 1999;54:594–604.
Middlemiss W, Granger DA, Goldberg WA. Response to “let’s help parents help themselves: a letter to the editor supporting the safety of behavioural sleep techniques”. Early Hum Dev. 2013;89(1):41–2.
Cohen J. Statistical power analysis for the behavioral science. 2nd ed. New York: Lawrence Erlbaum Associate; 1988.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863.
Cheng J, Edwards LJ, Maldonado-Molina MM, Komro KA, Muller KE. Real longitudinal data analysis for real people: building a good enough mixed model. Stat Med. 2010;29(4):504–20.
Maas CJM, Snijders TAB. The multilevel approach to repeated measures for complete and incomplete data. Qual Quant. 2003;37(1):71–89.
Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17(11):1261–91.
Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81.
Veldema J, Bosl K, Nowak DA. Motor recovery of the affected hand in subacute stroke correlates with changes of contralesional cortical hand motor representation. Neural Plasticity. 2017;2017:6171903.
Hommel M, Detante O, Favre I, Touze E, Jaillard A. How to measure recovery? Revisiting concepts and methods for stroke studies. Transl Stroke Res. 2016;7(5):388–94.
Rehme AK, Volz LJ, Feis DL, Eickhoff SB, Fink GR, Grefkes C. Individual prediction of chronic motor outcome in the acute post-stroke stage: behavioral parameters versus functional imaging. Hum Brain Mapp. 2015;36(11):4553–65.
Carey LM, Abbott DF, Egan GF, Bernhardt J, Donnan GA. Motor impairment and recovery in the upper limb after stroke: behavioral and neuroanatomical correlates. Stroke. 2005;36(3):625–9.
Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20(4):2166–80.
Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59(3):2771–82.
Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382(6594):805–7.
Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci. 2009;106(3):918–23.
Heddings AA, Friel KM, Plautz EJ, Barbay S, Nudo RJ. Factors contributing to motor impairment and recovery after stroke. Neurorehabil Neural Repair. 2000;14(4):301–10.
Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24(8):1000–19.
Boltze J, Lukomska B, Jolkkonen J, MEMS-IRBI Consortium. Mesenchymal stromal cells in stroke: improvement of motor recovery or functional compensation? J Cereb Blood Flow Metab. 2014;34(8):1420–1.
Mays RW, Savitz SI. Intravenous cellular therapies for acute ischemic stroke. Stroke. 2018;49(5):1058–65.
Foley N, McClure JA, Meyer M, Salter K, Bureau Y, Teasell R. Inpatient rehabilitation following stroke: amount of therapy received and associations with functional recovery. Disabil Rehabil. 2012;34(25):2132–8.
Acknowledgments
We thank the other members of the ISIS-HERMES Study group (listed in alphabetical order): S. Achard, P. Antoine, E. L. Barbier C.E. Bulabois, L. Carey, A. Chrispin, M. Cucherat, P. Davoine, F. de Fraipont, C. Delon-Martin, C. Dubray, H. Egelhofer, M.C. Favrot, K. Garambois, P. Garnier, J. Gere, N. Gonnet, I Goundous, F.F. Hannanu, O. Heck, A.V. Jaillard, A. Krainik, J.F. Le Bas, S. Miguel, A. B. Naegele, A. Paris, D. Perennou, P. Pernot, C. Remy, F. Renard, M.J. Richard, G. Rodier, E. Schir A. Thuriot, I. Tropres, and J. Warnking.
Trial Registration
ClinicalTrials.gov, number NCT00875654. https://clinicaltrials.gov/ct2/show/NCT00875654?term=ISIS+stroke+stem+cells&rank=1
Protocols
French ISIS RCT and satellite MRI HERMES protocols are available on demand.
Funding
This trial was funded by an academic grant from the French Health Ministry: PHRCI Grant numbers: ISIS-2007PHR04 and HERMES-2007-A00853-50. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. MRI data acquisition was performed at the IRMaGe MRI platform, which gratefully acknowledge financial support from France Life Imaging network through the grant “ANR-11-INBS-0006.” Data monitoring was performed by the Clinical Investigation Center (CIC) INSERM UMS 002 CHU Grenoble Alpes. Data analysis was partly supported by RESSTORE project (www.resstore.eu) funded by the European Commission under the H2020 program (Grant Number 681044).
Author information
Authors and Affiliations
Consortia
Contributions
Dr. Jaillard had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Concept and design: A. Jaillard, M. Hommel, and O. Detante. Acquisition of data. Recruitment and/or clinical follow-up: O. Detante, I. Favre-Wiki, M. Barbieux-Guillot, W. Vadot, and S. Marcel. MRI data acquisition: A. Jaillard, M. Hommel, L. Lamalle, and S. Grand. Analysis or interpretation of data: A. Jaillard, M. Hommel, T. A. Zeffiro, and O. Detante. Drafting of the manuscript: A. Jaillard, O. Detante, M. Hommel, T.A. Zeffiro, and A. Moisan. Critical revision of the manuscript for important intellectual content: A. Jaillard, T.A. Zeffiro, M. Hommel, and O. Detante. Statistical analysis: A. Jaillard and M. Hommel. Obtaining funding: A. Jaillard and O. Detante. Administrative, technical, or material support: A. Jaillard, M. Hommel, O. Detante, L. Lamalle (MRI calibration), and A. Moisan (Autologous mesenchymal stem cell preparation). Study supervision: O. Detante (ISIS) and A. Jaillard (HERMES).
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All patients gave written informed consent. The trial and the amendments were approved by the local ethics committee (“Comité de Protection des Personnes”). ISIS was monitored by an independent data and safety monitoring board (DSMB).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 2.06 mb)
Rights and permissions
About this article
Cite this article
Jaillard, A., Hommel, M., Moisan, A. et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl. Stroke Res. 11, 910–923 (2020). https://doi.org/10.1007/s12975-020-00787-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00787-z