Abstract
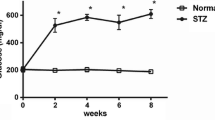
Diabetes is an important risk factor for ischemic stroke (IS). Tissue-type plasminogen activator (tPA) has been associated with less successful revascularization and poor functional outcome in diabetes. We assessed whether a new thrombolytic strategy based on MMP10 was more effective than tPA in a murine IS model of streptozotocin (STZ)-induced diabetes. Wild-type mice were administered a single dose of streptozotocin (STZ) (180 mg/kg) to develop STZ-induced diabetes mellitus. Two weeks later, IS was induced by thrombin injection into the middle cerebral artery and the effect of recombinant MMP10 (6.5 μg/kg), tPA (10 mg/kg) or tPA/MMP10 on brain damage and functional outcome were analysed. Motor activity was assessed using the open field test. Additionally, we studied plasminogen activator inhibitor-1 (PAI-1) and thrombin-antithrombin complex levels (TAT) by ELISA and oxidative stress and blood-brain barrier (BBB) integrity by immunohistochemistry and western blot. MMP10 treatment was more effective at reducing infarct size and neurodegeneration than tPA 24 h and 3 days after IS in diabetic mice. Locomotor activity was impaired by hyperglycemia and ischemic injury, but not by the thrombolytic treatments. Additionally, TAT, oxidative stress and BBB permeability were reduced by MMP10 treatment, whereas brain bleeding or PAI-1 expression did not differ between treatments. Thrombolytic treatment with MMP10 was more effective than tPA at reducing stroke and neurodegeneration in a diabetic murine model of IS, without increasing haemorrhage. Thus, we propose MMP10 as a potential candidate for the clinical treatment of IS in diabetic patients.






Similar content being viewed by others
References
Kearney K, Tomlinson D, Smith K, Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol. 2017;16(1):34. https://doi.org/10.1186/s12933-017-0515-9.
Tsujimoto T, Sugiyama T, Shapiro MF, Noda M, Kajio H. Risk of cardiovascular events in patients with diabetes mellitus on beta-blockers. Hypertension. 2017;70(1):103–10. https://doi.org/10.1161/HYPERTENSIONAHA.117.09259.
Papatheodorou K, Banach M, Edmonds M, Papanas N, Papazoglou D. Complications of diabetes. J Diabetes Res. 2015;2015:189525. https://doi.org/10.1155/2015/189525.
Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6(3):145–55. https://doi.org/10.1038/nrneurol.2009.231.
Lee SJ, Hong JM, Lee SE, Kang DR, Ovbiagele B, Demchuk AM, et al. Association of fibrinogen level with early neurological deterioration among acute ischemic stroke patients with diabetes. BMC Neurol. 2017;17(1):101. https://doi.org/10.1186/s12883-017-0865-7.
Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38(10):2771–8. https://doi.org/10.1161/STROKEAHA.107.487520.
Simao F, Ustunkaya T, Clermont AC, Feener EP. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood. 2017;129(16):2280–90. https://doi.org/10.1182/blood-2016-09-740670.
Dunn EJ, Philippou H, Ariens RA, Grant PJ. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia. 2006;49(5):1071–80. https://doi.org/10.1007/s00125-006-0197-4.
Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38(3):1044–9. https://doi.org/10.1161/01.STR.0000258041.75739.cb.
Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31(10):2325–9.
Xiang FL, Lu X, Strutt B, Hill DJ, Feng Q. NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice. Diabetes. 2010;59(10):2603–11. https://doi.org/10.2337/db09-1562.
Ceriello A, Novials A, Ortega E, Pujadas G, La Sala L, Testa R, et al. Hyperglycemia following recovery from hypoglycemia worsens endothelial damage and thrombosis activation in type 1 diabetes and in healthy controls. Nutr Metab Cardiovasc Dis. 2014;24(2):116–23. https://doi.org/10.1016/j.numecd.2013.05.003.
Fernandez-Cadenas I, Mendioroz M, Munuera J, Alvarez-Sabin J, Rovira A, Quiroga A, et al. Lower concentrations of thrombin-antithrombin complex (TAT) correlate to higher recanalisation rates among ischaemic stroke patients treated with t-PA. Thromb Haemost. 2009;102(4):759–64. https://doi.org/10.1160/TH08-06-0398.
Kota SK, Meher LK, Jammula S, Kota SK, Krishna SV, Modi KD. Aberrant angiogenesis: the gateway to diabetic complications. Indian J Endocrinol Metab. 2012;16(6):918–30. https://doi.org/10.4103/2230-8210.102992.
Coll B, Rodriguez JA, Craver L, Orbe J, Martinez-Alonso M, Ortiz A, et al. Serum levels of matrix metalloproteinase-10 are associated with the severity of atherosclerosis in patients with chronic kidney disease. Kidney Int. 2010;78(12):1275–80. https://doi.org/10.1038/ki.2010.329.
Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50(4):329–39. https://doi.org/10.1002/glia.20169.
Toni M, Hermida J, Goni MJ, Fernandez P, Parks WC, Toledo E, et al. Matrix metalloproteinase-10 plays an active role in microvascular complications in type 1 diabetic patients. Diabetologia. 2013;56(12):2743–52. https://doi.org/10.1007/s00125-013-3052-4.
Rodriguez JA, Orbe J, Martinez de Lizarrondo S, Calvayrac O, Rodriguez C, Martinez-Gonzalez J, et al. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci. 2008;13:2916–21.
Orbe J, Barrenetxe J, Rodriguez JA, Vivien D, Orset C, Parks WC, et al. Matrix metalloproteinase-10 effectively reduces infarct size in experimental stroke by enhancing fibrinolysis via a thrombin-activatable fibrinolysis inhibitor-mediated mechanism. Circulation. 2011;124(25):2909–19. https://doi.org/10.1161/CIRCULATIONAHA.111.047100.
Rodriguez JA, Sobrino T, Lopez-Arias E, Ugarte A, Sanchez-Arias JA, Vieites-Prado A, et al. CM352 Reduces brain damage and improves functional recovery in a rat model of intracerebral hemorrhage. J Am Heart Assoc. 2017;6(6) https://doi.org/10.1161/JAHA.117.006042.
Roncal C, Martinez de Lizarrondo S, Salicio A, Chevilley A, Rodriguez JA, Rosell A, et al. New thrombolytic strategy providing neuroprotection in experimental ischemic stroke: MMP10 alone or in combination with tissue-type plasminogen activator. Cardiovasc Res. 2017;113(10):1219–29. https://doi.org/10.1093/cvr/cvx069.
Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37(6):1399–406. https://doi.org/10.1161/01.STR.0000223001.06264.af.
Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal. 2013;18(12):1400–17. https://doi.org/10.1089/ars.2012.4721.
Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. https://doi.org/10.1161/STR.0b013e318284056a.
Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5(4):442–53. https://doi.org/10.1007/s12975-014-0336-z.
Bruno A, Liebeskind D, Hao Q, Raychev R, Investigators US. Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol. 2010;12(6):492–503. https://doi.org/10.1007/s11940-010-0093-6.
Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43(2):567–70. https://doi.org/10.1161/STROKEAHA.111.635250.
European Stroke Organisation Executive C, Committee ESOW. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457–507. https://doi.org/10.1159/000131083.
Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. https://doi.org/10.1124/pr.57.2.4.
Abdelsaid M, Prakash R, Li W, Coucha M, Hafez S, Johnson MH, et al. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes. 2015;64(5):1804–17. https://doi.org/10.2337/db14-1423.
Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8(1):82–9. https://doi.org/10.1016/j.coph.2007.12.001.
Ning R, Chopp M, Yan T, Zacharek A, Zhang C, Roberts C, et al. Tissue plasminogen activator treatment of stroke in type-1 diabetes rats. Neuroscience. 2012;222:326–32. https://doi.org/10.1016/j.neuroscience.2012.07.018.
Orset C, Haelewyn B, Allan SM, Ansar S, Campos F, Cho TH, et al. Efficacy of alteplase in a mouse model of acute ischemic stroke: a retrospective pooled analysis. Stroke. 2016;47(5):1312–8. https://doi.org/10.1161/STROKEAHA.116.012238.
Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603.
Inzitari D, Giusti B, Nencini P, Gori AM, Nesi M, Palumbo V, et al. MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke. 2013;44(10):2901–3. https://doi.org/10.1161/Strokeaha.113.002274.
Kowluru RA, Mohammad G, dos Santos JM, Zhong Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011;60(11):3023–33. https://doi.org/10.2337/db11-0816.
Desilles JP, Syvannarath V, Ollivier V, Journe C, Delbosc S, Ducroux C, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke. 2017;48(7):1932–40. https://doi.org/10.1161/STROKEAHA.117.017080.
Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69(14):2345–63. https://doi.org/10.1007/s00018-012-1011-8.
Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32(9):3044–57. https://doi.org/10.1523/JNEUROSCI.6409-11.2012.
Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44(2):130–9. https://doi.org/10.1007/s12031-011-9496-4.
Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9(9):1180–6. https://doi.org/10.1038/nm911.
Ou Z, Tao MX, Gao Q, Zhang XL, Yang Y, Zhou JS, et al. Up-regulation of angiotensin-converting enzyme in response to acute ischemic stroke via ERK/NF-kappaB pathway in spontaneously hypertensive rats. Oncotarget. 2017;8(57):97041–51. https://doi.org/10.18632/oncotarget.21156.
Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41(2–3):172–9. https://doi.org/10.1007/s12035-010-8102-z.
Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI, Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. The Biochemical Journal. 2016;473(24):4527–50. https://doi.org/10.1042/BCJ20160503C.
Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64(6):654–63. https://doi.org/10.1002/ana.21511.
Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, et al. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107(5):1196–205. https://doi.org/10.1111/j.1471-4159.2008.05664.x.
Miller AA, Dusting GJ, Roulston CL, Sobey CG. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res. 2006;1111(1):111–6. https://doi.org/10.1016/j.brainres.2006.06.082.
Ribo M, Montaner J, Molina CA, Arenillas JF, Santamarina E, Alvarez-Sabin J. Admission fibrinolytic profile predicts clot lysis resistance in stroke patients treated with tissue plasminogen activator. Thromb Haemost. 2004;91(6):1146–51. https://doi.org/10.1160/TH04-02-0097.
Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat. 2016;30(4):738–45. https://doi.org/10.1016/j.jdiacomp.2015.12.018.
Acknowledgements
We thank Lara Montori for the help with the experimental work and Rosa Tordera and Mikel Aleixo from Pharmacology and Toxicology Department, University of Navarra for their technical assistance with behavioural tests.
Funding
This work was supported by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III [FIS PI15/01807] and Federal Ministry of Education and Research (FEDER) funds; grants from the Spanish Society of Thrombosis and Haemostasis (SETH), Navarra Government (02/2015), Patrimonio Praga (Mexico) and Virto Group (Navarra, Spain); and PhD scholarship from the Asociación de Amigos de la Universidad de Navarra (ADA).
Author information
Authors and Affiliations
Contributions
MNO participated in the design of the project, experimental work, statistical analysis, and wrote, reviewed, and edited the manuscript; CR participated in the design of the project and reviewed the manuscript. AS was in charge of the animal experiments; MB performed histological studies; OR participated in the design of the project and reviewed the manuscript; ET participated in the statistical analysis and reviewed the manuscript; BZ participated in the design of the project and reviewed the manuscript; JAR participated in the design of the project and reviewed the manuscript; JAP participated in the design of the project, supervised the work, and edited and reviewed the manuscript; RM participated in the design of the project and edited and reviewed the manuscript; JO was in charge of the whole project design, supervised the work, and wrote, edited, and reviewed this manuscript.
Corresponding author
Ethics declarations
Experiments were performed in accordance with European Communities Council Directive guidelines (2010/63/EU) for the care and use of laboratory animals and were approved by the University of Navarra Animal Research Review Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal experiments were performed in accordance with European Communities Council Directive guidelines (2010/63/EU) for the care and use of laboratory animals and were approved by the University of Navarra Animal Research Review Committee.
Rights and permissions
About this article
Cite this article
Navarro-Oviedo, M., Roncal, C., Salicio, A. et al. MMP10 Promotes Efficient Thrombolysis After Ischemic Stroke in Mice with Induced Diabetes. Transl. Stroke Res. 10, 389–401 (2019). https://doi.org/10.1007/s12975-018-0652-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0652-9