Abstract
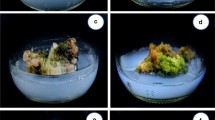
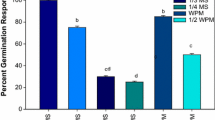
The aim of this study is to introduce the suitable protocol for indirect regeneration from seedling-derived leaf segment of Ficus religiosa. The leaf explant successfully produced callus on MS medium containing various concentrations of auxin in combination with BAP. The maximum callus induction (100%) was achieved in MS medium containing 0.5 mg/l 2,4-D plus 0.05 mg/l BAP and MS medium containing 1.5 mg/l NAA plus 0.15 mg/l BAP as well. MS medium consisting of 2,4-D produced yellow-brownish and friable callus (type I) while the yellowish and compact calli (type II) were obtained in MS medium consisting of NAA. On the other hand, MS medium supplemented with IBA formed greenish and compact calli (type Ш). The regeneration rate in type II callus was less than the type I, and there was no shoot induction observed on type Ш calli. MS medium supplemented with 1.5 mg/l BAP in combination with 0.15 mg/l IBA had the highest regeneration frequency (100%) and maximum shoot numbers (5.16) as well as shoot length (2.56 cm) in type I callus. A maximum of 93.33% root induction was observed in MS medium supplemented with 2.0 mg/l IBA plus 0.1mg/l NAA. The plantlets were successfully transferred to the greenhouse. This system could be utilized for large-scale multiplication of Ficus religiosa.
Similar content being viewed by others
References
Ahmad N, Anis M. 2007. Rapid clonal multiplication of a woody tree, Vitex negundo L. through axillary shoots proliferation. Agrofor. Syst. 71: 195–200
Angulo-Bejarano PI, Paredes-López O. 2011. Development of a regeneration protocol through indirect organogenesis in prickly pear cactus (Opuntia ficus-indica (L.) Mill). Sci. Hort. 128: 283–288
Arab MM, Yadollahi A, Shojaeiyan A, Shokri S, Ghojah SM. 2014. Effects of nutrient media, different cytokinin types and their concentrations on in vitro multiplication of G× N15 (hybrid of almond× peach) vegetative rootstock. J. Genet. Eng. Biotechnol. 12: 81–87
Bhangale JO, Acharya NS, Acharya SR. 2016. Protective effect of Ficus religiosa (L.) against 3-nitropropionic acid induced Huntington disease. Orient. Pharm. Exp. Med. 16: 165–174
Bhojwani SS, Dantu PK. 2013. Micropropagation. In: S. S. Bhojwani and P. K. Dantu, editors, Plant Tissue Culture: An Introductory Text. Springer, New Delhi, pp 245–274
Bosela MJ, Michler C. 2008. Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinin types. In Vitro Cell Dev. Biol. Plant 44: 316–329
Cagno V, Civra A, Kumar R, radhan S, Donalisio M, Sinha BN, et al. 2015. Ficus religiosa L. bark extracts inhibit human rhinovirus and respiratory syncytial virus infection in vitro. J. Ethnopharmacol. 176: 252–257
Chen Y, Zhang Y, Cheng Q, Niu M, Liang H, Yan H, et al. 2016. Plant regeneration via direct and callus-mediated organogenesis from leaf explants of Chirita swinglei (Merr.) WT Wang. In Vitro Cell Dev. Biol. Plant 52: 521–529
Çördük N, Aki C. 2011. Inhibition of browning problem during micropropagation of Sideritis trojana bornm., an endemic medicinal herb of Turkey. Rom. Biotech. Lett. 16: 6761–6765
Deshpande S, Josekutty P, Prathapasenan G. 1998. Plant regeneration from axillary buds of a mature tree of Ficus religiosa. Plant Cell Rep. 17: 571–573
Feng J.-C, Yu X, Shang X, Li J, Wu Y. 2010. Factors influencing efficiency of shoot regeneration in Ziziphus jujuba Mill.‘Huizao’. Plant Cell Tissue Organ Cult. 101: 111–117
Garcia R, Pacheco G, Falcão E, Borges G, Mansur E. 2011. Influence of type of explant, plant growth regulators, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa L. (Passifloraceae). Plant Cell Tissue Organ Cult. 106: 47–54
Ge F, Luo X, Huang X, Zhang Y, He X, Liu M, et al. 2016. Genome-wide analysis of transcription factors involved in maize embryonic callus formation. Physiol. Plant 158: 452–462
Ghosh M, Civra A, Rittà M, Cagno V, Mavuduru SG, Awasthi P, et al. 2016. Ficus religiosa L. bark extracts inhibit infection by herpes simplex virus type 2 in vitro. Arch. Virol. 161: 3509–3514
Hassan AS, Afroz F, Jahan MAA, Khatun R. 2009. In vitro regeneration through apical and axillary shoot proliferation of Ficus religiosa l.-a multi-purpose woody medicinal plant. Plant Tissue Cult. Biotechnol. 19: 71–78
Hesami M, Daneshvar MH. 2016a. Development of a regeneration protocol through indirect organogenesis in Chenopodium quinoa Wild. Indo-Am. J. Agric. Vet. Sci. 4: 25–32
Hesami M, Daneshvar MH. 2016b. Regeneration from callus which is produced from cotyledon of Antirrhinum majus. Indo-Am. J. Agric. Vet. Sci. 4: 20–24
Hesami M, Daneshvar MH. 2018. In vitro adventitious shoot regeneration through direct and indirect organogenesis from seedling-derived hypocotyl segments of Ficus religiosa L.: an important medicinal plant. HortScience. 53: 55–61
Hesami M, Daneshvar MH, Lotfi A. 2017a. In vitro shoot proliferation through cotyledonary node and shoot tip explants of Ficus religiosa L. Plant Tissue Cult. Biotech. 27: 85–88
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M. 2018a. An efficient in vitro shoot regeneration through direct organogenesis from seedling-derived petiole and leaf segments and acclimatization of Ficus religiosa. J. For. Res.
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M. 2018b. Establishment of a protocol for in vitro seed germination and callus formation of Ficus religiosa L., an important medicinal plant. Jundishapur J. Nat. Pharm. Prod. 13: e62682
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M, Alizadeh M. 2017b. Effect of plant growth regulators on indirect shoot organogenesis of Ficus religiosa through seedling derived petiole segments. J. Genet. Eng. Biotech.
Hesami M, Naderi R, Yoosefzadeh-Najafabadi M, Rahmati M. 2017c. Data-driven modeling in plant tissue culture. J. Appl. Environ. Biol. Sci. 7: 37–44
Jafari M, Daneshvar MH, Lotfi A. 2017. In vitro shoot proliferation of Passiflora caerulea L. via cotyledonary node and shoot tip explants. BioTechnologia 98: 113–119
Jaiswal V, Narayan P. 1985. Regeneration of plantlets from the callus of stem segments of adult plants of Ficus religiosa L. Plant Cell Rep. 4: 256–258
Karami O, Piri K, Bahmani R. 2009. Plant regeneration through callus cultures derived from immature-cotyledon explants of oleaster (Elaeagnus angustifolia L.). Trees 23: 335–338
Keshari AK, Kumar G, Kushwaha PS, Bhardwaj M, Kumar P, Rawat A, et al. 2016. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J. Ethnopharmacol. 181: 252–262
Kirana H, Agrawal S, Srinivasan B. 2009. Aqueous extract of Ficus religiosa Linn. reduces oxidative stress in experimentally induced type 2 diabetic rats. Indian J. Exp. Biol. 47: 822–826
Ko W, Su C, Chen C, Chao C. 2009. Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tissue Organ Cult. 96: 137–141
Lavanya A, Muthukrishnan S, MuthuKumar M, Benjamin JF, Kumar TS, Kumaresan V, et al. 2014. Indirect organogenesis from various explants of Hildegardia populifolia (Roxb.) Schott & Endl.–A threatened tree species from Eastern Ghats of Tamil Nadu, India. J. Genet. Eng. Biotechnol. 12: 95–101
Mali AM, Chavan NS. 2016. In vitro rapid regeneration through direct organogenesis and ex-vitro establishment of Cucumis trigonus Roxb.—An underutilized pharmaceutically important cucurbit. Ind. Crops Prod. 83: 48–54
Mansouri K, Preece JE. 2009. The influence of plant growth regulators on explant performance, bud break, and shoot growth from large stem segments of Acer saccharinum L. Plant Cell Tissue Organ Cult. 99: 313
Murthy KSR, Kondamudi R, Vijayalakshmi V. 2010. Micropropagation of an endangered medicinal plant Ceropegia spiralis L. Int. J. Agric. Technol. 6: 179–191
Mutawila C, Vinale F, Halleen F, Lorito M, Mostert L. 2016. Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathology 65: 104–113
Niazian M, Noori SAS, Galuszka P, Tohidfar M, Mortazavian SMM. 2017. Genetic stability of regenerated plants via indirect somatic embryogenesis and indirect shoot regeneration of Carum copticum L. Ind. Crops Prod. 97: 330–337
Pan Z, Zhu S, Guan R, Deng X. 2010. Identification of 2, 4-D-responsive proteins in embryogenic callus of Valencia sweet orange (Citrus sinensis Osbeck) following osmotic stress. Plant Cell Tissue Organ Cult. 103: 145–153
Pandey, N., R.P. Meena, and S.K. Rai S. Pandey-Rai. 2016. In vitro generation of high artemisinin yielding salt tolerant somaclonal variant and development of SCAR marker in Artemisia annua L. Plant Cell Tissue Organ Cult. 127: 301–314
Parasharami V, Yadav P, Mandkulkar S, Gaikwad S. 2014. Ficus religiosa L.: Callus, suspension culture and lectin activity in fruits and in vitro regenerated tissues. British Biotechnol. J. 4: 215–227
Patil MS, Patil C, Patil S, Jadhav R. 2011. Anticonvulsant activity of aqueous root extract of Ficus religiosa. J. Ethnopharmacol. 133: 92–96
Pawar PL, Nabar BM. 2010. Effect of plant extracts formulated in different ointment bases on MDR strains. Indian J. Pharm. Sci. 72: 397–401
Phulwaria M, Shekhawat N, Rathore J, Singh R. 2013. An efficient in vitro regeneration and ex vitro rooting of Ceropegia bulbosa Roxb.—a threatened and pharmaceutical important plant of Indian Thar Desert. Ind. Crops Prod. 42: 25–29
Sáenz L, Herrera-Herrera G, Uicab-Ballote F, Chan JL, Oropeza C. 2010. Influence of form of activated charcoal on embryogenic callus formation in coconut (Cocos nucifera). Plant Cell Tissue Organ Cult. 100: 301–308
Salmi MS, Hesami M. 2016. Time of collection, cutting ages, auxin types and concentrations influence rooting Ficus religiosa L. stem cuttings. J. Appl. Environ. Biol. Sci. 6: 124–132
Sharma S, Shahzad A, Mahmood S, Saeed T. 2015. High-frequency clonal propagation, encapsulation of nodal segments for short-term storage and germplasm exchange of Ficus carica L. Trees 29: 345–353
Singh C, Raj SR, Jaiswal P, Patil V, Punwar B, Chavda J, et al. 2016. Effect of plant growth regulators on in vitro plant regeneration of sandalwood (Santalum album L.) via organogenesis. Agrofor. Syst. 90: 281–288
Singh D, Singh B, Goel RK. 2011. Traditional uses, phytochemistry and pharmacology of Ficus religiosa: A review. J. Ethnopharmacol. 134: 565–583
Siwach P, Gill AR. 2011. Enhanced shoot multiplication in Ficus religiosa L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiol. Mol. Biol. Plants 17: 271
Siwach P, Gill AR. 2014. Micropropagation of Ficus religiosa L. via leaf explants and comparative evaluation of acetylcholinesterase inhibitory activity in the micropropagated and conventionally grown plants. 3 Biotech 4: 477–491
Siwach P, Gill AR, Kumari K. 2011. Effect of season, explants, growth regulators and sugar level on induction and long term maintenance of callus cultures of Ficus religiosa L. Afr. J. Biotechnol. 10: 4879–4886
Thaniarasu R, Kumar TS, Rao M. 2016. Mass propagation of Plectranthus bourneae Gamble through indirect organogenesis from leaf and internode explants. Physiol. Mol. Biol. Plants 22: 143–151
Thomas TD. 2008. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 26: 618–631
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hesami, M., Daneshvar, M.H. Indirect Organogenesis through Seedling-Derived Leaf Segments of Ficus Religiosa - a Multipurpose Woody Medicinal Plant. J. Crop Sci. Biotechnol. 21, 129–136 (2018). https://doi.org/10.1007/s12892-018-0024-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-018-0024-0